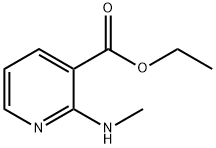
ethyl 2-(methylamino)pyridine-3-carboxylate synthesis
- Product Name:ethyl 2-(methylamino)pyridine-3-carboxylate
- CAS Number:103976-61-0
- Molecular formula:C9H12N2O2
- Molecular Weight:180.2
Yield: 100%
Reaction Conditions:
in tetrahydrofuran at 40; for 3 h;
Steps:
5.1.23. Ethyl 2-(methylamino)nicotinate (30)
To a mixture of 29 (1.0 g, 5.4 mmol) in THF (10 ml) was added methylamine (0.66 ml, 6.5 mmol) at rt. The mixture was stirred at 40 °C for 3 h. The mixture was concentrated in vacuo. The residue was purified by column chromatography (silica gel, eluted with 050% AcOEt in hexane) to give 30 (1.0 g, quantative yield) as a colorless solid. 1 H NMR (300 MHz, CDCl3) δ 1.30-1.45 (3H, m), 3.06 (3H, d, J = 4.8 Hz), 4.32 (2H, q, J = 7.2 Hz), 6.51 (1H, dd, J = 7.7, 4.8 Hz), 7.92 (1H, brs), 8.11 (1H, dd, J = 7.7, 2.0 Hz), 8.31 (1H, dd, J = 4.8, 1.9 Hz). LCMS (ESI+) m/z 181.1.
References:
Adachi, Ryutaro;Amano, Nobuyuki;Ishichi, Yuji;Kamaura, Masahiro;Kubo, Kazuki;Matsunaga, Nobuyuki;Miyamoto, Yasufumi;Nakahata, Takashi;Nakakariya, Masanori;Nishimura, Satoshi;Oikawa, Tatsuo;Okamoto, Rei;Satomi, Yoshinori;Yukawa, Takafumi [Bioorganic and medicinal chemistry,2020]
2942-59-8
818 suppliers
$5.00/25g

103976-61-0
11 suppliers
inquiry

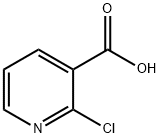
64-17-5
736 suppliers
$10.00/50g
32399-13-6
65 suppliers
$60.00/100mg

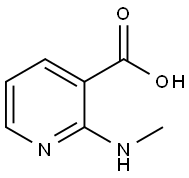
103976-61-0
11 suppliers
inquiry

