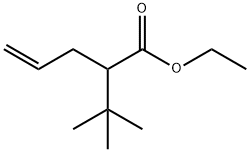
Ethyl 2-tert-Butylpent-4-enoate synthesis
- Product Name:Ethyl 2-tert-Butylpent-4-enoate
- CAS Number:122936-14-5
- Molecular formula:C11H20O2
- Molecular Weight:184.28
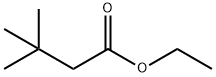
5340-78-3
87 suppliers
$24.00/5g
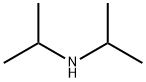
108-18-9
390 suppliers
$10.00/1g

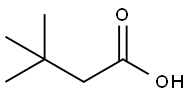
106-95-6
430 suppliers
$10.00/5g

122936-14-5
5 suppliers
inquiry
Yield:-
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane;
Steps:
7.i (i)
(i) Preparation of ethyl (RS)-2-(1,1-dimethylethyl)pent-4-enoate. n Butyllithium (44 cm3 of a 2.5 molar solution in Hexane) was added to a solution of dry di-isopropylamine (14.7 cm3) in dry tetrahydrofuran (75 cm3) whilst the temperature was maintained at -40°C. The reaction mixture was then allowed to warm to 0°C before being cooled to -70°C. A solution of ethyl 3,3-dimethylbutanoate (14.4 g) in dry tetrahydrofuran (20 cm3) was then added dropwise to the reaction mixture which was maintained at -70°C. After the addition was complete, the reaction temperature was allowed to rise to -60°C for 15 minutes, before being cooled again to -70°C. A solution of allyl bromide (13.4 g) in dry tetrahydrofuran (5 cm3) was then added in portions to the reaction mixture, which was allowed to warm to the ambient temperature, and stirred for a further 16 hours. The solvent was removed by evaporation under reduced pressure and the residual liquid was poured into water, and extracted into diethyl ether. The combined organic extracts were washed with water, dilute aqueous hydrochloric acid and water again. The organic portion was dried over anhydrous magnesium sulphate, and concentrated by evaporation of the solvent under reduced pressure. The residual liquid was subjected to fractional distillation under reduced pressure through a Vigreux column to give ethyl (RS)-2-(1,1-dimethylethyl)pent-4-enoate (14.3 g), boiling point 49-50°C/0.6 mmHg. 90 MHz 1H NMR (CDCl3): 0.95 (9H,s): 1.20 (3H,t); 2.20 (3H,m); 4.10 (2H,q); 4.7-5.8 (3H,m). Infra Red (Liquid film): 3083, 2966, 1729, 1641, 1477, 1369, 1346, 1151, 1028 and 915 cmmin1
References:
EP295839,1991,A3
1070-83-3
290 suppliers
$27.00/25mL

122936-14-5
5 suppliers
inquiry
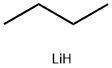
29786-93-4
0 suppliers
inquiry

5340-78-3
87 suppliers
$24.00/5g

108-18-9
390 suppliers
$10.00/1g

106-95-6
430 suppliers
$10.00/5g

122936-14-5
5 suppliers
inquiry