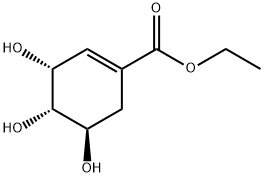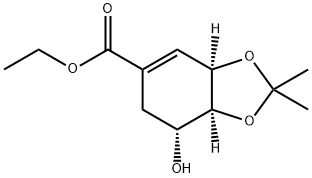
Ethyl 3,4-O-isopropylideneshikiMate synthesis
- Product Name:Ethyl 3,4-O-isopropylideneshikiMate
- CAS Number:136994-78-0
- Molecular formula:C12H18O5
- Molecular Weight:242.27
Yield: 88%
Reaction Conditions:
with toluene-4-sulfonic acid in ethyl acetate under 112.511 - 150.015 Torr; for 2 h;
Steps:
1 Example 1
Shikimic acid 3 (50g, 0.36mol) was added to absolute ethanol (100ml), and sulfoxide chloride (9.3ml, 6.9mmol) was added dropwise with stirring.After the addition is complete, heat to reflux for 3 hours, and distill off the solvent under reduced pressure to obtain ethyl shikimate.Ethyl acetate (300ml) was added, followed by 2,2-dimethoxypropane (26.7g, 0.257mol),P-toluenesulfonic acid monohydrate (0.5g, 2.6mmol), 150-200 mbar vacuum distillation reaction, after 2 hours,The volume of the reactant was distilled off by 50%, 2,2-dimethoxypropane (26.7g, 0.257mol) was added, and the distillation reaction under reduced pressure was continued. After 2 hours,The raw material disappeared, the reaction was stopped, and after cooling to room temperature, it was washed with saturated sodium bicarbonate (80ml) and saturated brine (80ml).It was dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and n-heptane was recrystallized to obtain the target compound 5 (53 g, 88%).
References:
Beijing Rong Ying Pharmaceutical Technology Co., Ltd.;Lu Jianxun;Ye Luding;Wang Ying CN108218697, 2020, B Location in patent:Paragraph 0029-0032
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

136994-78-0
25 suppliers
inquiry

138-59-0
590 suppliers
$5.00/250mg

136994-78-0
25 suppliers
inquiry
![(3aR,4R,7S,8aR)-Tetrahydro-7-hydroxy-2,2-dimethyl-4,7-methano-1,3-dioxolo[4,5-c]oxepin-6(4H)-one](/CAS/20200611/GIF/32384-42-2.gif)
32384-42-2
1 suppliers
inquiry

136994-78-0
25 suppliers
inquiry