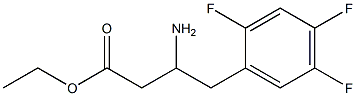
ethyl 3-amino-4-(2,4,5-trifluorophenyl)butanoate synthesis
- Product Name:ethyl 3-amino-4-(2,4,5-trifluorophenyl)butanoate
- CAS Number:1151240-92-4
- Molecular formula:C12H14F3NO2
- Molecular Weight:261.2403
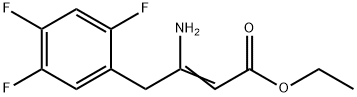
1151240-89-9
2 suppliers
inquiry

1151240-92-4
4 suppliers
inquiry
Yield:1151240-92-4 56%
Reaction Conditions:
Stage #1: 3-amino-4-(2,4,5-trifluorophenyl)-but-2-enoic acid ethyl esterwith sodium tetrahydroborate;acetic acid at 15 - 25; for 1 h;
Stage #2: with sodium hydrogencarbonate in water; pH=10 - 11;
Steps:
5
Sodium borohydride (1.12 g, 0.03 mol) was added carefully with portions to acetic acid at 150C to 200C (strong, exothermic reaction). 3-amino-4-(2,4,5- trifluorophenyl)but-2-enoic acid ethyl ester (2.6 g, 0.01 mol) was added to the prepared mixture at 2O0C, and the resulting mixture was stirred for 1 hour at 20°C to 25 0C. Acetic acid was evaporated, the residue was dissolved in methylene chloride (100 mL), and washed with an aqueous saturated solution OfNaHCO3 to pH 10-11. The organic layer was dried over Na2SO4, filtered, and evaporated to give yellowish oil (1.45 g, 56 % yield). The oil was treated with a solution of HCl/EtOH and evaporated to give yellowish oil (1.7 g), which solidified with time
References:
WO2009/64476,2009,A1 Location in patent:Page/Page column 15
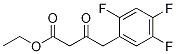
1151240-88-8
37 suppliers
inquiry

1151240-92-4
4 suppliers
inquiry
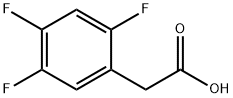
209995-38-0
483 suppliers
$10.00/10g

1151240-92-4
4 suppliers
inquiry
![2,2-DiMethyl-5-[(2,4,5-trifluorophenyl)acetyl]-1,3-dioxane-4,6-dione](/CAS/20150408/GIF/1152439-73-0.gif)
1152439-73-0
7 suppliers
inquiry

1151240-92-4
4 suppliers
inquiry