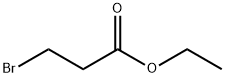
Ethyl 3-(benzyloxy)propanoate synthesis
- Product Name:Ethyl 3-(benzyloxy)propanoate
- CAS Number:127113-02-4
- Molecular formula:C12H16O3
- Molecular Weight:208.25
Yield:127113-02-4 77%
Reaction Conditions:
Stage #1: 3-benzyloxypropan-1-olwith Jones reagent in acetone at 0 - 20; for 2 h;Jones Oxidation;
Stage #2: ethanolwith sulfuric acid for 12 h;Reflux;
Steps:
4 Example 4; Scheme for Synthesizing Compound 4:
Compound 1 (10 g, 60.2 mmol) was dissolved into 200 ml acetone. At 0° C., Jones reagent (26.72 g chromium trioxide: 23 ml concentrated sulfuric acid, diluted with water to 100 ml) was added dropwise until the orange red color was sustained without turning green. The temperature returned to the room temperature followed by agitation for 2 h. The resultant mixture was vacuum suck filtrated and loaded onto a column which was eluted with acetone. After acetone was removed by vacuum rotatory evaporation, the organic phases were extracted with ethyl acetate for three times. The organic phases were combined and washed once with saturated saline. After dried with anhydrous sodium sulfate and rotatory evaporation, 100 ml ethanol and 2 ml concentrated H2SO4 were added for 12 h reflux reaction. After most solvent was removed by rotatory evaporation, water/ethyl acetate phase separation was conducted. The organic phase was washed with sodium bicarbonate solution, water, and saturated saline respectively, and then dried with anhydrous sodium sulfate. After the solvent was removed by rotatory evaporation, reduced pressure distillation was conducted to obtain 9.7 g colorless oily liquid 4 with a yield of 77%. 1H NMR (CDCl3, 400 MHz, ppm) δ: 7.36-7.28 (m, 5H), 4.54 (s, 2H), 4.17-4.13 (q, J=7.0 Hz, 2H), 3.77-3.74 (t, J=6.2 Hz, 2H), 2.63-2.60 (t, J=6.2 Hz, 2H), 1.28-1.24 (t, J=7.1 Hz, 3H).
References:
US2016/2164,2016,A1 Location in patent:Paragraph 0118-0120

64-17-5
706 suppliers
$10.00/50g
27912-85-2
73 suppliers
$58.00/250mg

127113-02-4
11 suppliers
inquiry

4799-68-2
177 suppliers
$5.00/1g

127113-02-4
11 suppliers
inquiry

100-44-7
627 suppliers
$13.50/250G

127113-02-4
11 suppliers
inquiry