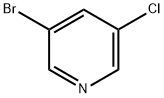
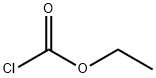
Ethyl 3-broMo-5-chloroisonicotinate synthesis
- Product Name:Ethyl 3-broMo-5-chloroisonicotinate
- CAS Number:1214387-79-7
- Molecular formula:C8H7BrClNO2
- Molecular Weight:264.5
Yield:1214387-79-7 85%
Reaction Conditions:
Stage #1: 3-bromo-5-chloropyridinewith lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 0.75 h;Inert atmosphere;
Stage #2: chloroformic acid ethyl ester in tetrahydrofuran;n-heptane;ethylbenzene; for 0.583333 h;
Stage #3: with sodium hydrogencarbonate in tetrahydrofuran;n-heptane;ethylbenzene;Product distribution / selectivity;
Steps:
102; 103; 147
To a solution of LDA (2 M solution in THF/heptane/ethylbenzene, 2.78 g, 12.97 mL, 25.95 mmol) in anhydrous THF (20 mL) under nitrogen atmosphere cooled to -78°C was added a solution of the 3-bromo-5-chloropyridine (5.0 g, 25.98 mmol) in anhydrous THF (40 mL) at -78°C. The reaction mixture was allowed to stir at the same temperature for 45 minutes. Then, a solution of chloro(ethoxy)methanone (28.19 g, 259.7 mmol) was added slowly over 15 minutes. After stirring for 20 minutes, the reaction mixture was quenched with saturated NaHCO3 solution. The reaction mixture was extracted into ethyl acetate (3 x 100 mL), and the combined organic layer was washed with water and brine. The organic layer was separated, dried over anhydrous sodium sulfate, and filtered. The filtrate was evaporated, and the residue was purified through CombiFlash using 0-10% ethyl acetate in hexane to provide ethyl 3-bromo-5-chloropyridine-4-carboxylate as a pale yellow oil (5.84 g, 85% yield); 1H NMR (400 MHz, DMSO-J6): δ 1.35 (t, 3H), 4.45 (q, 2H), 8.82 (s, IH), 8.87 (s, IH); M+ 265.5.
References:
WO2010/132999,2010,A1 Location in patent:Page/Page column 161; 163; 212