
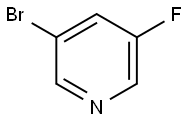
Ethyl 3-Bromo-5-Fluoroisonicotinate synthesis
- Product Name:Ethyl 3-Bromo-5-Fluoroisonicotinate
- CAS Number:1214335-25-7
- Molecular formula:C8H7BrFNO2
- Molecular Weight:248.05
407-20-5
339 suppliers
$10.00/1g
541-41-3
6 suppliers
$21.00/5g

1214335-25-7
18 suppliers
$45.00/50mg
Yield:1214335-25-7 670 mg
Reaction Conditions:
Stage #1: 3-bromo-5-fluoropyridinewith lithium diisopropyl amide in tetrahydrofuran at -78; for 1 h;
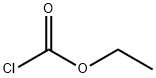
Stage #2: chloroformic acid ethyl ester in tetrahydrofuran at -78 - 20; for 1 h;
Steps:
91 Synthesis of 3-(8-carbamoyl-5,6,7,8-tetrahydro-[1,8]naphthyridin-3-yl)-5-fluoro-isonicotinic acid methyl ester (Cpd 211, Table 1)
To a round bottom flask is added 3-bromo-5-fluoropyridine (475 mg, 2.7 mmol) in 4 ml of dry THF at -78° C., followed by the addition of lithium diisopropylamine (1.62 ml, 3.24 mmol). The reaction mixture is stirred at -78° C. for 1 hour, followed by the addition of ethyl chloroformate (586 mg, 5.4 mmol). The reaction mixture is warmed up to room temperature and stirred for 1 hour. The reaction mixture is diluted with EtOAc, washed with saturated NH4Cl/water, brine, dried over anhydrous Na2SO4, filtered and concentrated to give the crude product. Purification by the flash column chromatography affords 670 mg of 3-bromo-5-fluoro-isonicotinic acid ethyl ester.
References:
US2014/323468,2014,A1 Location in patent:Paragraph 0676