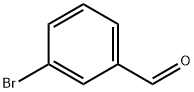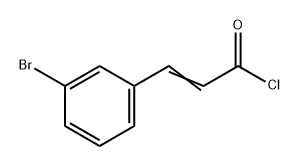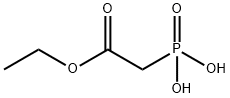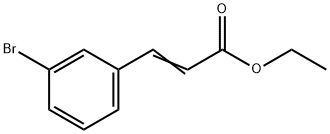
ethyl 3-bromo-cinnamate synthesis
- Product Name:ethyl 3-bromo-cinnamate
- CAS Number:24398-80-9
- Molecular formula:C11H11BrO2
- Molecular Weight:255.11
Yield:24398-80-9 98 %
Reaction Conditions:
in dichloromethane at 0 - 20;Inert atmosphere;
Steps:
142 Synthesis of 130
To a sthed solution of 1 O7a (150 mg, 0.308 mmo1 LOO equiv) and (3S)-3- fluoropyrrolidine hydrochloride (115.93 mg, 0,924 mmol, 3 equiv) in T)CE (5 mL) were added TEA (93.42 mg, 0S24 mrnol, 3 equiv) at room temperature under nitrogen atmosphere. The resulting mixture was stirred for Iii at room temperature. To the above mixture was added STAB (130.44 mg. 06i6 mmoi, 2 equiv) at room temperature. The resulting mixture was stirred for additional 3h at room temperature. The reaction was quenched by the addition of NH4.C1 (aq.) (20 mL) at room temperature. The aqueous layer was extracted with DCM (2x20 niL), The crude product (100 mg) was purified by Prep HPLC with the foflowing conditions (Column: XBridge Prep 0131) Ci 8 Column, 30*150 mm, 5arn; Mobile Phase A: Water( 10 rnrnol/L NH4HCO3), Mobile Phase B: ACN; Flow rate:60 rnL/rnin; Gradient: 25% B to 45% B in 7 mint Wave Length: 220 rim; RTI(rnin): 630) to afford 109 (33.7 mg, 1924%) as a yeflow solid.LC-MS: (ES, m/z): [M+H] 561H-NMR: (400 MHz, CD3OD, Sppm): 1.99-2.11 (m, 1H), 2.18-2.25 (m, 1H), 2.47-2.51 (m, 1H), 2.69-2.8 1 (m, 1H), 2.89-2.98 (m, 2H), 3.01 (s, 3H), 3.51 (s, 2H), 3.66 (s, 2H), 3.78 (s, 3H), 5.06 (s, 4H), 5.12-5.26 (m, 1H), 6.38 (s, 1H), 6.85-6.86 (m, 1H), 7.11-7.13 (m, 2H),7.25-7.26 (m, 1H), 7.70(s, 1H), 8.23 (s, 1H).
References:
WO2022/221704,2022,A1 Location in patent:Paragraph 512
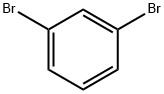
108-36-1
444 suppliers
$10.00/1g
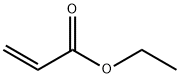
140-88-5
515 suppliers
$12.00/100ml

24398-80-9
20 suppliers
inquiry