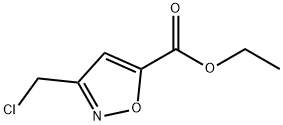
Ethyl 3-(chloroMethyl)isoxazole-5-carboxylate synthesis
- Product Name:Ethyl 3-(chloroMethyl)isoxazole-5-carboxylate
- CAS Number:1141427-74-8
- Molecular formula:C7H8ClNO3
- Molecular Weight:189.6

82934-25-6
0 suppliers
inquiry

623-47-2
338 suppliers
$10.00/1g

1141427-74-8
25 suppliers
$306.00/1g
Yield: 35%
Reaction Conditions:
with sodium hypochlorite in tetrahydrofuran at 0 - 20; for 18 h;
Steps:
22.2
To a solution of (lE)-2-chloroacetaldehyde oxime (4.0 g, 42.8 mmol) in tetrahydrofuran (15 mL) was added ethyl prop-2 -ynoate (4.2 g, 42.8 mmol) and sodium hypochlorite (181.5 g, 243.8 mmol, 10% purity) at 0 °C. After stirred at 20 °C for 18 hours, the reaction mixture was diluted with ethyl acetate (200 mL) and brine (200 mL). The separated organic layer was dried over sodium sulphate and concentrated to dryness under reduced pressure. The residue was purified by column chromatography (silica gel, 100 - 200 mesh, 0 - 10% ethyl acetate in petroleum ether) to afford ethyl 3-(chloromethyl)isoxazole-5-carboxylate (2.8 g, 35%) as a white solid.
References:
TENAYA THERAPEUTICS, INC. WO2021/127643, 2021, A1 Location in patent:Paragraph 00630-00631
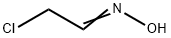
51451-05-9
16 suppliers
inquiry

623-47-2
338 suppliers
$10.00/1g

1141427-74-8
25 suppliers
$306.00/1g
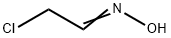
51451-05-9
16 suppliers
inquiry

623-47-2
338 suppliers
$10.00/1g

1141427-74-8
25 suppliers
$306.00/1g

1379165-76-0
0 suppliers
inquiry
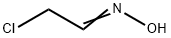
51451-05-9
16 suppliers
inquiry
105-37-3
385 suppliers
$10.00/5mL

1141427-74-8
25 suppliers
$306.00/1g