
ethyl 3-(dibenzylaMino)-2,2-difluoropropanoate synthesis
- Product Name:ethyl 3-(dibenzylaMino)-2,2-difluoropropanoate
- CAS Number:541547-36-8
- Molecular formula:C19H21F2NO2
- Molecular Weight:333.37

667-27-6
409 suppliers
$10.00/10g
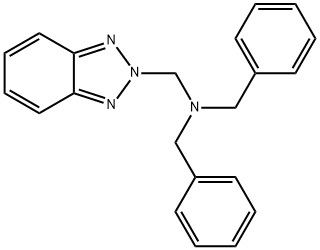
121238-95-7
0 suppliers
inquiry
![N-((1H-benzo[d][1,2,3]triazol-1-yl)Methyl)-N-benzyl-1-phenylMethanaMine](/CAS/GIF/57684-32-9.gif)
57684-32-9
25 suppliers
$77.00/250mg

541547-36-8
32 suppliers
$122.00/100mg
Yield:541547-36-8 99%
Reaction Conditions:
Stage #1: Ethyl bromodifluoroacetatewith chloro-trimethyl-silane;zinc in tetrahydrofuran at 20; for 0.166667 h;
Stage #2: 2H-1,2,3-benzotriazol-2-yl-N,N-dibenzylmethanamine;N-((1 H-benzo[d][1,2,3]triazol-1-yl)methyl)-N-benzyl-1-phenylmethanamine in tetrahydrofuran at 20; for 3 h;
Steps:
To a suspension of zinc powder (SIGMA-ALDRICH, 3.22 g, 49.2 mmol) in THF (96 mL), at rt, was added TMSCI (SIGMA-ALDRICH, 3.14 mL, 24.60 mmol). The mixture was stirred for 10 min and then ethyl bromodifluoroacetate (FLUOROCHEM, 3.48 mL, 27.1 mmol) was added. After stirring at rt for 10 min, a solution of mixture of 1 H- and 2H-1 ,2,3-benzotriazol-1-yl-N,N- dibenzylmethanamine (8.08 g, 24.60 mmol) in THF (27.3 mL) was added and the reaction mixture was stirred at rt for 3 h. A saturated solution of NaHCOs sat. was added, and the mixture was stirred for 15 min and filtered through a plug of Celite washing with EtOAc. Layers were separated, and the aqueous layer was extracted with EtOAc two times. The combined organic layers were dried, filtered and the solvent was removed in vacuo to obtain ethyl 3- (dibenzylamino)-2,2-difluoropropanoate (8.1 1 g, 99% yield). This material was used without further purification. NMR (300 MHz, CDCI3) d ppm: 7.39-7.26 (m, 10H), 4.19 (q, J= 7.1 Hz, 2H), 3.70 (s, 4H), 3.16 (t, J= 13.1 Hz, 2H), 1.24 (t, J= 7.1 Hz, 3H).
References:
WO2019/145360,2019,A1 Location in patent:Page/Page column 37; 38

667-27-6
409 suppliers
$10.00/10g
![N-((1H-benzo[d][1,2,3]triazol-1-yl)Methyl)-N-benzyl-1-phenylMethanaMine](/CAS/GIF/57684-32-9.gif)
57684-32-9
25 suppliers
$77.00/250mg

541547-36-8
32 suppliers
$122.00/100mg
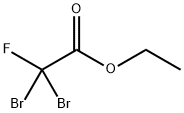
565-53-7
117 suppliers
$45.00/5G
![N-((1H-benzo[d][1,2,3]triazol-1-yl)Methyl)-N-benzyl-1-phenylMethanaMine](/CAS/GIF/57684-32-9.gif)
57684-32-9
25 suppliers
$77.00/250mg

541547-36-8
32 suppliers
$122.00/100mg
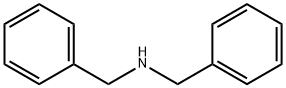
103-49-1
437 suppliers
$5.00/25g

541547-36-8
32 suppliers
$122.00/100mg
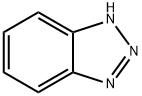
95-14-7
857 suppliers
$6.00/100g

50-00-0
846 suppliers
$10.00/25g

667-27-6
409 suppliers
$10.00/10g

103-49-1
437 suppliers
$5.00/25g

541547-36-8
32 suppliers
$122.00/100mg