
Ethyl 3-fluoro-4-(trifluoromethyl)phenylacetate synthesis
- Product Name:Ethyl 3-fluoro-4-(trifluoromethyl)phenylacetate
- CAS Number:1255041-77-0
- Molecular formula:C11H10F4O2
- Molecular Weight:250.19
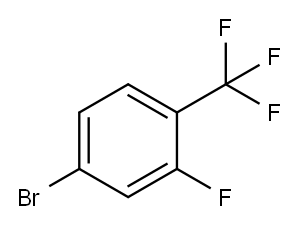
142808-15-9
227 suppliers
$6.00/1g
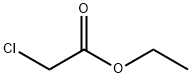
105-39-5
391 suppliers
$10.00/5g

1255041-77-0
1 suppliers
inquiry
Yield:1255041-77-0 27%
Reaction Conditions:
with manganese;(2,2'-bipyridine)nickel(II) dibromide;trifluoroacetic acid in N,N-dimethyl-formamide at 65; for 1 h;Inert atmosphere;
Steps:
6.6.5.a
In a 50 mL two necked round bottom flask 4-bromo-2-fluoro-1-(trifluoromethyl)benzene (0.5g, 2.05 mmol), ethyl chloroacetate (328 mg, 2.67 mmol), and dimethylformamide (4 mL) were charged. The system was degassed and re filled with argon followed by addition of Mn (225 mg, 4.1 mmol) and NiBr2. bipy (62 mg, 0.16 mmol). Finally TFA (4.1 μL) was added and the reaction mixture was degasified and refilled with argon. Then it was heated to 650C for one hour. TLC showed (10% ethyl acetate in hexane, Rf: 0.2) complete conversion of starting material. The reaction mixture was diluted with water (50 mL) and HCI (4N, 0.5 mL) and extracted with ethyl acetate (3 x 40 mL). The combined organic part was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to afford crude material which was purified by column chromatography (silica gel: 100-200 mesh, eluent: 10% ethyl acetate in hexane) to afford the pure compound (490 mg, 27%). 1H NMR (DMSOd6, 400 MHz): δ 7.54 (t, 1H), 7.15 (d, 2H), 4.16 (q, 2H), 3.64 (s, 2H), 1.26 (t, 3H).
References:
WO2010/127856,2010,A1 Location in patent:Page/Page column 88