
ETHYL 3-FLUORO-5-NITROBENZOATE synthesis
- Product Name:ETHYL 3-FLUORO-5-NITROBENZOATE
- CAS Number:850807-07-7
- Molecular formula:C9H8FNO4
- Molecular Weight:213.16

850807-07-7
17 suppliers
inquiry
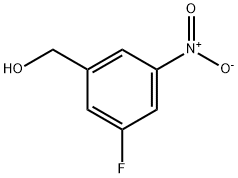
883987-74-4
23 suppliers
$153.00/1g
Yield:883987-74-4 74%
Reaction Conditions:
Stage #1: ethyl 3-fluoro-5-nitrobenzoatewith lithium borohydride in tetrahydrofuran;diethyl ether at 20; for 2 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;diethyl ether;water;
Steps:
34.a
REFERENCE EXAMPLE 34; 5-Fluoro-{3-methylsuIfonyImethy.)phenylamine; a) 5-F.uoro-(3-hydroxymethy.)-1-nitrobenzene; 1 ,29 ml_ of 2.0 N BH4Li solution in THF (2.58 mmol) were added to a soiution of ethyl 3-fiuoro-5-nitrobenzoate (50Qmg, 2.35 mmol) in Et2O (5 mL). The reaction mixture was stirred at room temperature for 2h. The crude product was diluted in1 N HCI aqueous solution and the aqueous phase was extracted thrice with Et2O.The combined organic phases were separated, dried over Na2SO4, and evaporated to dryness. The crude product thus obtained was purified by column chromatography over siiica gel using hexane/EtOAc mixtures of increasing polarity as eluent and 355 mg (2.07 mmol) of the desired compound were obtained (yieid:74%).
References:
WO2010/34740,2010,A1 Location in patent:Page/Page column 93

850807-07-7
17 suppliers
inquiry
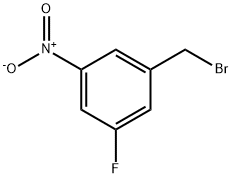
883987-75-5
13 suppliers
inquiry

