ethyl (3-formyl-2-methyl-1H-indol-1-yl)acetate synthesis
- Product Name:ethyl (3-formyl-2-methyl-1H-indol-1-yl)acetate
- CAS Number:433307-59-6
- Molecular formula:C14H15NO3
- Molecular Weight:245.27
Yield:433307-59-6 86%
Reaction Conditions:
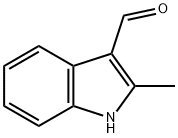
Stage #1: 2-methyl-1H-indole-3-carbaldehydewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;
Stage #2: ethyl bromoacetate in tetrahydrofuran;mineral oil at 0 - 20; for 3.5 h;
Steps:
5 Example 5 Synthesis of ethyl 2-(3-formyl-2-1H-indol-1-yl)acetate (6)
2-methyl-1H-indole-3-carbaldehyde (5) (3 g, 18.8 mmol) in tetrahydrofuran (THF) (50 mL) was cooled to 0° C. in an ice bath and mixed with sodium hydride (NaH) (60% dispersion in mineral oil) (1.5 g, 37.6 mmol). The mixture was stirred at 0° C. for 30 minutes, to which ethyl bromoacetate (2.5 mL, 28.6 mmol) was added. The resulting mixture was stirred at 0° C. for 30 minutes, warmed at room temperature, and further stirred for 3 hours. At this time, the TLC analysis showed the consumption of the starting material. The reaction mixture was cooled and further cooled down with ice water. The THF solvent was removed under vacuum, and the residue was diluted with H2O and ethyl acetate. After phase separation, the aqueous layer was extracted with ethyl acetate (3×200 mL), and the combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated under vacuum. The concentrate was recrystallized from diethyl ether and hexane to yield a purified product of ethyl 2-(3-formyl-2-1H-indol-1-yl)acetate (6) as a pale yellow solid (4.0 g, yield 86%). The 1H NMR spectrum of the resulting ethyl 2-(3-formyl-2-1H-indol-1-yl)acetate (6) is shown in FIG. 9, and the mass spectrum is shown in FIG. 10. 1H NMR (400 MHz, DMSO-d6) δ ppm: 10.125 (1H, s, -CHO), 8.125-8.103 (1H, dd, J=7.6, 2.8, Ar-H), 7.530-7.508 (1H, dd, J=6.8, 2, Ar-H), 7.255-7.200 (2H, m, Ar-H), 5.236 (2H, s, -N-CH2), 4.208-4.155 (2H, q, J=7.2, -O-CH2), 2.652 (3H, s, -CH3), 1.241-1.205 (3H, t, J=7.2, -CH3); ESIMS found: m/z 244.4 [M-H]-, 246.4 [M+H]+, 268.4 [M+Na]+, Rf=0.53 (hexane:ethyl acetate=2:1).
References:
US2016/102078,2016,A1 Location in patent:Paragraph 0076-0079
95-20-5
395 suppliers
$5.00/10g

433307-59-6
17 suppliers
$23.00/1g