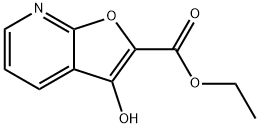
ETHYL 3-HYDROXYFURO[2,3-B]PYRIDINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 3-HYDROXYFURO[2,3-B]PYRIDINE-2-CARBOXYLATE
- CAS Number:109274-83-1
- Molecular formula:C10H9NO4
- Molecular Weight:207.18

623-50-7
260 suppliers
$5.00/1g

1452-94-4
247 suppliers
$15.00/10g
![ETHYL 3-HYDROXYFURO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/109274-83-1.gif)
109274-83-1
20 suppliers
$130.00/100mg
Yield:109274-83-1 94%
Reaction Conditions:
Stage #1: ethyl 2-hydroxyacetatewith sodium hydride in 1,2-dimethoxyethane;mineral oil at 0 - 23; for 0.5 h;
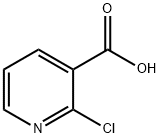
Stage #2: ethyl 2-chloronicotinate in 1,2-dimethoxyethane;mineral oil at 70; for 15.1667 h;
Steps:
104.1 104.1 Ethyl 3-hyd roxyfuro[2,3-b]pyrid ine-2-carboxylate
A suspension of sodium hydride (11.2 g, 60% dispersion in mineral oil, 280 mmol) in1 ,2-dimethoxyethane (250 mL) was cooled to 000, treated dropwise with ethyl glycolate(25.5 mL, 269 mmol) and stirred at 23 00 for 30 mi Ethyl-2-chloronicotinate (20.0 g, 108 mmol) in 1,2-dimethoxyethane (40 mL) was added dropwise over 10 mm and the mixture was stirred at 70 00 for 15 hours. The solvent was evaporated, the residue dissolved in water (500 mL) and washed with toluene. The aqueous layer was acidifiedwith acetic acid (19 mL) to pH Sand extracted five times with CH2CI2 (5 x 100 mL). The combined organic layers were dried over anhydrous MgSO4, filtered and the solvent evaporated. Column chromatography (Si02 EtOAc/Heptane, 20:80 ->50:50) of the crude gave ethyl 3-hydroxyfuro[2,3-b]pyridine-2-carboxylate (21.1 g, 94%) as a yellow solid.1H NMR (400 MHz, Chloroform-o) 6 = 8.52 (dd, J= 4.9, 1.7 Hz, 1H, H-Ar), 8.12 (dd, J7.8, 1.7 Hz, 1H, H-Ar), 7.31 (dd, J= 7.8, 4.8 Hz, 1H, H-Ar), 4.47 (q, J= 7.1 Hz, 2H, 0-CH2CH3), 4.13 (s, 1H, OH), 1.44 (t, J= 7.1 Hz, 3H, 0-CH2CH3) ppm.MS (ESI+, H20/MeCN) mlz(%): 208.0 (100, [M + H]j.
References:
WO2018/229193,2018,A1 Location in patent:Page/Page column 134; 135

1452-94-4
247 suppliers
$15.00/10g
![ETHYL 3-HYDROXYFURO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/109274-83-1.gif)
109274-83-1
20 suppliers
$130.00/100mg
2942-59-8
788 suppliers
$5.00/25g
![ETHYL 3-HYDROXYFURO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/109274-83-1.gif)
109274-83-1
20 suppliers
$130.00/100mg