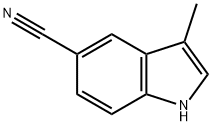
ethyl 3-methyl-1H-indole-5-carboxylate synthesis
- Product Name:ethyl 3-methyl-1H-indole-5-carboxylate
- CAS Number:73396-90-4
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
Yield:73396-90-4 73%
Reaction Conditions:
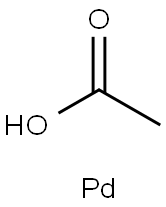
with triethanolamine;tetrakis(triphenylphosphine)palladium (0) in acetonitrile;
Steps:
5.1 (1)
(1) Synthesis of 5-Carboethoxy-3-methylindole To a solution of 2-bromo-4-carboethoxy-N-allylaniline (J. Org. Chem., 45, 2710 (1980)) (650 mg, 2.29 mmol) in acetonitrile (10 ml), tetrakis(triphenylphosphine)palladium(0) (132 mg, 0.114 mmol), palladium (II) acetate (25.7 mg, 0.114 mmol) and TEA (0.413 ml, 2.98 mmol) were added and stirred overnight in a sealed tube at 100° C. After filtering the reaction mixture, the filtrate was evaporated under reduced pressure; the thus obtained residue was subjected to silica gel column chromatography (developing solvent: ethyl acetate:n-hexane=1:2) to give the titled compound (340 mg, 73%). NMR (method a, CDCl3): δ 1.43 (3H, t, J=7.2 Hz), 2.37 (3H, s), 4.41 (2H, q, J=7.2 Hz), 7.03 (1H, s), 7.34 (1H, d, J=8.6 Hz), 7.91 (1H, dd, J=1.3, 8.6 Hz), 8.08 (1H, brs), 8.36 (1H, s)
References:
US6586630,2003,B1
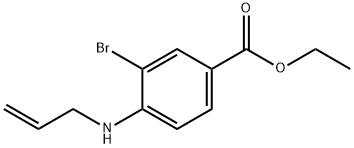
73396-89-1
0 suppliers
inquiry

73396-90-4
33 suppliers
inquiry
17380-18-6
104 suppliers
$13.00/1g

73396-90-4
33 suppliers
inquiry
3613-06-7
24 suppliers
inquiry

73396-90-4
33 suppliers
inquiry

64-17-5
709 suppliers
$10.00/50g
588688-44-2
45 suppliers
$45.00/10mg

73396-90-4
33 suppliers
inquiry