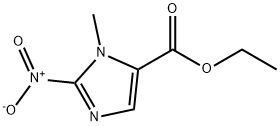
Ethyl 3-Methyl-2-nitro-3H-iMidazole-4-carboxylate synthesis
- Product Name:Ethyl 3-Methyl-2-nitro-3H-iMidazole-4-carboxylate
- CAS Number:39070-13-8
- Molecular formula:C7H9N3O4
- Molecular Weight:199.16

177760-04-2
108 suppliers
$26.00/100mg

39070-13-8
46 suppliers
inquiry
Yield: 75%
Reaction Conditions:
with acetic acid;sodium nitrite in water at -5 - 20;
Steps:
4.1.1.4. Ethyl 1-N-methyl-2-nitro-1H-imidazole-5-carboxylate (5).
To a solution of sodium nitrite (18.3 g, 266 mmol) in water (55 mL)cooled around 5 °C in an ice-salt bath, was added dropwise asolution of the amino ester 4 (6.42 g, 38.0 mmol) in acetic acid (42 mL). The temperature was allowed to rise gradually to rt and the reaction mixture was stirred overnight. The reaction mixturewas extracted with dichloromethane (3 50 mL). The combined organic layers were dried over MgSO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel using cyclohexane/ethyl acetate (7/3,v/v) as eluent. After concentration under vacuum, the residue was washed with water (2 50 mL). The combined organic layers were dried over MgSO4, filtered and evaporated under reduced pressure to yield nitro ester 5 as yellow crystals (5.67 g, 28.5 mmol). Yield 75%; mp 56-58 °C (Lit. 56-58 °C [40] and 65-66 °C [37]); Rf 0.40(SiO2, cyclohexane/ethyl acetate, 7/3, v/v); IR (ATR) n cm1 1723,1552, 1486, 1519, 1364, 1233, 767; 1H NMR (CDCl3, 300 MHz) δ 7.70(s, 1H, CHAr), 4.39 (q, 2H, 3J 7.1 Hz, OCH2CH3), 4.31 (s, 3H, NCH3),1.39 (t, 3H, 3J 7.1 Hz, OCH2CH3); 13C NMR (DMSO-d6, 126 MHz) δ 158.71 (CO), 147.61 (CArNO2), 133.70 (CHAr), 126.07 (CArCO), 61.34(OCH2), 35.14 (NCH3), 13.91 (CH2CH3).
References:
Ghedira, Donia;Voissière, Aurélien;Peyrode, Caroline;Kraiem, Jamil;Gerard, Yvain;Maubert, Elise;Vivier, Magali;Miot-Noirault, Elisabeth;Chezal, Jean-Michel;Farhat, Farhat;Weber, Valérie [European Journal of Medicinal Chemistry,2018,vol. 158,p. 51 - 67]

89531-66-8
11 suppliers
inquiry

39070-13-8
46 suppliers
inquiry