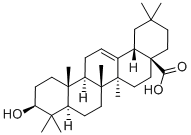
Ethyl (3beta)-3-hydroxyolean-12-en-28-oate synthesis
- Product Name:Ethyl (3beta)-3-hydroxyolean-12-en-28-oate
- CAS Number:110700-49-7
- Molecular formula:C32H52O3
- Molecular Weight:484.75
Yield: 97%
Reaction Conditions:
Stage #1:Oleanolic acid with potassium carbonate in N,N-dimethyl-formamide for 0.5 h;
Stage #2:ethyl iodide in N,N-dimethyl-formamide at 20; for 12 h;
Steps:
(3β) Ethyl 3-hydroxyolean-12-en-28-oate (6)
Oleanolic acid (10.0 g, 0.022 mol) was dissolved in DMF (200 mL) and potassium carbonate(15.0 g, 0.110 mol) was added. The mixture was stirred for 30 min, and ethyl iodide (3.5 mL,0.044 mol) was added. After stirring for 12 h at room temperature, the product was precipitated by adding water (800 mL). The product was filtered off and washed with 2 M hydrochloric acid(2 20 mL), water (2 20 mL); drying in a desiccator gave 6 as a colorless crystalline solid (10.3 g,97%). m.p. = 175-178 °C (Lit.: 217.5-218.0 C) [29]; [α]20D = +73.4 (c 0.34, CHCl3) (lit.: +78.9) [29];Rf = 0.68 (silica gel, hexane/EtOAc, 8:2); IR (KBr): = 3446br, 2946m, 2868w, 1722m, 1636w, 1462w,1386w, 1364w, 1260w, 1178m, 1162m, 1124w, 1094w, 1064w, 1036m cm1; 1H NMR (500 MHz, CDCl3): = 5.28 (dd, J = 3.6, 3.6 Hz, 1H, H-12), 4.08 (qq, J = 10.8, 7.1 Hz, 2H, H-31), 3.20 (m, 1H, H-3), 2.86(dd, J = 13.9, 4.3 Hz, 1H, H-18), 2.00-1.91 (m, 1H, H-16a), 1.90-1.80 (m, 2H, H-11a + H-11b), 1.73-1.55(m, 7H, H-22a + H-19a + H-1a + H-15a + H-2a + H-2b + H-16b), 1.54-1.40 (m, 4H, H-6a + H-22b +H-9 + H-7a), 1.40-1.24 (m, 3H, H-6b + H-21a + H-7b), 1.22 (t, J = 7.1 Hz, 3H, H-32), 1.20-1.10 (m,2H, H-21b + H-19b), 1.13 (s, 3H, H-27), 1.10-1.00 (m, 1H, H-15b), 0.98 (s, 3H, H-24), 0.98-0.93 (m, 1H,H-1b), 0.92 (s, 3H, H-30), 0.90 (s, 3H, H-25), 0.89 (s, 3H, H-29), 0.77 (s, 3H, H-23), 0.74 (s, 3H, H-26),0.71 (m, 1H, H-5) ppm; 13C NMR (125 MHz, CDCl3): = 177.8 (C-28), 144.0 (C-13), 122.4 (C-12),79.2 (C-3), 60.2 (C-31), 55.4 (C-5), 47.79 (C-9), 46.7 (C-17), 46.1 (C-19), 41.9 (C-14), 41.5 (C-18), 39.5 (C-8),38.9 (C-1), 38.6 (C-4), 37.2 (C-10), 34.1 (C-21), 33.3 (C-29), 32.9, (C-7), 32.6 (C-22), 30.8 (C-20), 28.3 (C-24),27.8 (C-15), 27.4 (C-2), 26.0 (C-27), 23.8 (C-30), 23.6(C-11), 23.2 (C-16), 18.5 (C-6), 17.1 (C-26), 15.7 (C-23),15.5 (C-25), 14.4 (C-32) ppm; MS (ESI, MeOH): m/z (%) = 467.3 ([M H2O + H]+, 70), 480.2 ([M + H]+,74), 991.5 ([2M + Na]+, 100).
References:
Serbian, Immo;Csuk, René [Molecules,2018,vol. 23,# 7,art. no. 1552]