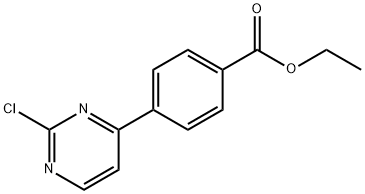
ethyl 4-(2-chloropyriMidin-4-yl)benzoate synthesis
- Product Name:ethyl 4-(2-chloropyriMidin-4-yl)benzoate
- CAS Number:499195-60-7
- Molecular formula:C13H11ClN2O2
- Molecular Weight:262.69
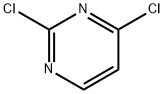
3934-20-1
705 suppliers
$9.00/1g
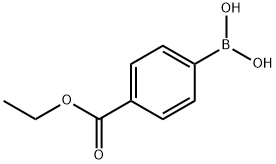
4334-88-7
280 suppliers
$6.00/1g

499195-60-7
42 suppliers
$37.00/100mg
Yield:499195-60-7 60%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in toluene at 75 - 100;Suzuki Coupling;
Steps:
1
Example 1 - Synthesis of Compound 3; A mixture of 4-ethoxycarbonylphenyl boronic acid (23.11 g, 119 mmol), 2,4- dichloropyrimidine (16.90 g, 113 mmol), toluene (230 mL) and aqueous sodium carbonate (2 M, 56 mL) was stirred vigorously and nitrogen was bubbled through the suspension for 15 minutes. Tetrakis(triphenylphosphine)palladium[0] (2.61 g, 2.26 mmol) was added. Nitrogen was bubbled through for another 10 min., the mixture was heated to 100°C, then at 75°C overnight. The mixture was cooled, diluted with ethyl acetate (200 mL), water (100 mL) was added and the layers were separated. The aqueous layer was extracted with ethyl acetate (100 ml) and the two organic extracts were combined. The organics were washed with brine, filtered through sodium sulfate,
References:
WO2008/109943,2008,A1 Location in patent:Page/Page column 56-57