
Ethyl 4,5-dimethyl-1,3-thiazole-2-carboxylate synthesis
- Product Name:Ethyl 4,5-dimethyl-1,3-thiazole-2-carboxylate
- CAS Number:79247-88-4
- Molecular formula:C8H11NO2S
- Molecular Weight:185.24
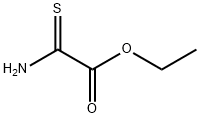
16982-21-1
233 suppliers
$6.00/1g
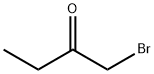
816-40-0
118 suppliers
$45.00/50mg

79247-88-4
23 suppliers
$130.00/50mg
Yield:79247-88-4 77%
Reaction Conditions:
in ethanol; for 1.41 h;Reflux;
Steps:
398.1 Ethyl 4-ethylthiazole-2-carboxylate, Example 398.1
A suspension of ethyl 2-thiooxamate (Sigma-Aldrich, 5.05 g, 37.9 mmol) in EtOH (32 mL) was heated to reflux. Once all of the solids dissolved, 1-bromo-2-butanone (Sigma-Aldrich, 3.9 mL, 37.2 mmol) was added slowly via syringe to the refluxing solution over a 10 min period. The resulting orange solution was heated at reflux for an additional 1.25 h and then was cooled to RT. The reaction mixture was poured into a beaker of ice water and then treated with ammonium hydroxide until strongly basic. The mixture was extracted with EtOAc (1X) and the organic layer was washed with brine (1X), dried over anhydrous sodium sulfate, and concentrated in vacuo. The residue was purified by fractional distillation under vacuum (bp = 140 °C-145 °C 1 mm Hg) to provide Example 398.1 (5.31 g, 77% yield) as a light yellow oil. LCMS-ESI (pos.) m/z: 186.2 (M+H)+.
References:
WO2018/97945,2018,A1 Location in patent:Paragraph 0687