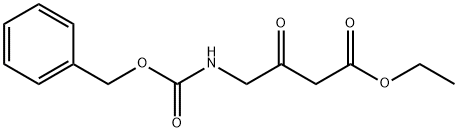
ETHYL 4-(BENZYLOXYCARBONYLAMINO)-3-OXOBUTANOATE synthesis
- Product Name:ETHYL 4-(BENZYLOXYCARBONYLAMINO)-3-OXOBUTANOATE
- CAS Number:67706-69-8
- Molecular formula:C14H17NO5
- Molecular Weight:279.29

6148-64-7
386 suppliers
$5.00/10g
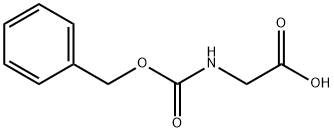
1138-80-3
447 suppliers
$6.00/10g

67706-69-8
14 suppliers
$165.00/0.25g
Yield:67706-69-8 60%
Reaction Conditions:
Stage #1: N-(Benzyloxycarbonyl)glycinewith 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 2.5 h;
Stage #2: ethyl potassium malonatewith magnesium chloride in tetrahydrofuran at 50; for 48 h;Cooling with ice;
Steps:
158A Example 158A
Ethyl 4-{[(benzyloxy)carbonyl] amino}-3-oxobutanoate
To a solution of 15.0 g (71.7 mmol) of N-[(benzyloxy)carbonyl]glycine in 534 ml of THF were added 9.24 g (57.0 mmol) of carbonyldiimidazole (CDI), and the mixture was stirred at RT for 2.5 h.
Subsequently, while cooling with an ice bath, 9.76 g (57.4 mmol) of potassium 3-ethoxy-3-oxopropanoate and 4.95 g (52.0 mmol) of magnesium chloride were added.
On completion of addition, stirring was continued at 50° C. for a further 48 h.
The solvent was removed under reduced pressure, the residue was taken up with ethyl acetate and saturated aqueous ammonium chloride solution, and the phases were separated.
The organic phase was washed with saturated aqueous sodium chloride solution, dried over sodium sulphate and filtered, and the solvent was removed under reduced pressure.
The residue was purified by means of normal phase chromatography (ethyl acetate-cyclohexane gradient), and 12.7 g (60% of theory; 95% purity) of the title compound were obtained.
LC-MS (Method 1): Rt=0.83 min; MS (ESIneg): m/z=278 [M-H]-
1H NMR (400 MHz, DMSO-d6): δ [ppm]=7.56 (br t, 1H), 7.25-7.41 (m, 5H), 5.04 (s, 2H), 4.09 (q, 2H), 3.97 (d, 2H), 3.60 (s, 2H), 1.19 (t, 3H).
References:
US2018/297994,2018,A1 Location in patent:Paragraph 1362-1365

1138-80-3
447 suppliers
$6.00/10g

67706-69-8
14 suppliers
$165.00/0.25g

6148-64-7
386 suppliers
$5.00/10g
![Carbamic acid, [2-(1H-imidazol-1-yl)-2-oxoethyl]-, phenylmethyl ester (9CI)](/CAS/20210305/GIF/22586-32-9.gif)
22586-32-9
0 suppliers
inquiry

67706-69-8
14 suppliers
$165.00/0.25g
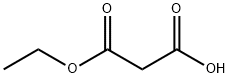
1071-46-1
286 suppliers
$5.00/1g
![Carbamic acid, [2-(1H-imidazol-1-yl)-2-oxoethyl]-, phenylmethyl ester (9CI)](/CAS/20210305/GIF/22586-32-9.gif)
22586-32-9
0 suppliers
inquiry

67706-69-8
14 suppliers
$165.00/0.25g