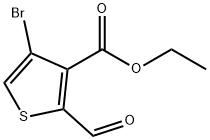
Ethyl 4-broMo-2-forMylthiophene-3-carboxylate synthesis
- Product Name:Ethyl 4-broMo-2-forMylthiophene-3-carboxylate
- CAS Number:1433203-92-9
- Molecular formula:C8H7BrO3S
- Molecular Weight:263.11
Yield:1433203-92-9 63.5%
Reaction Conditions:
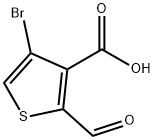
Stage #1: 4-bromo-2-formyl-thiophene-3-carboxylic acidwith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.166667 h;
Stage #2: ethyl iodide at 50; for 3 h;
Steps:
3.2 3.2 4-Bromo-2-formyl-thiophene-3-carboxylic acid ethyl ester
3.2
4-Bromo-2-formyl-thiophene-3-carboxylic acid ethyl ester
4-Bromo-2-formyl-thiophene-3-carboxylic acid (0.16 g, 0.68 mmol), K2CO3 (0.188 g, 1.36 mmol), and 4 mL of anhydrous DMF were stirred at room temperature for 10 min, then iodoethane was added (0.128 g, 0.8 mmol) dropwise.
The reaction solution was stirred at 50° C. for 3 h.
The reaction mixture was cooled, extracted with EA (3*50 mL), concentrated and the residue was purified by TLC (PE/EA=8/1) to give 0.4 g of the title compound (yield: 63.5%).
LC-MS: m/e (M+H)+: 263.7, Rt: 0.88 min.
References:
US2013/116233,2013,A1 Location in patent:Paragraph 1041; 1042
16694-17-0
163 suppliers
$11.00/250mg

1433203-92-9
6 suppliers
inquiry