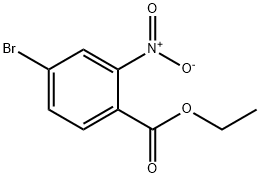
ethyl 4-bromo-2-nitrobenzoate synthesis
- Product Name:ethyl 4-bromo-2-nitrobenzoate
- CAS Number:528872-23-3
- Molecular formula:C9H8BrNO4
- Molecular Weight:274.07

64-17-5
705 suppliers
$10.00/50g
99277-71-1
226 suppliers
$9.00/5g

528872-23-3
14 suppliers
$192.24/250mg
Yield:528872-23-3 89%
Reaction Conditions:
with sulfuric acidCooling with ice;Reflux;
Steps:
Ethyl 4-bromo-2-nitrobenzoate (3b).
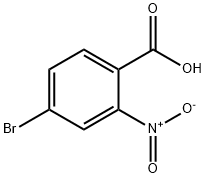
The compound has been previously reported.7 4-bromo-2-nitrobenzoic acid (2b, 24.0 g, 97.7 mmol)was dissolved in ethanol (200 mL) and cooled on an ice-water bath. Concentrated H2SO4 was addedin portions to the cooled solution over 10 min. The reaction mixture was then heated at reflux for 22h before it was cooled to room temperature. Most of the ethanol was removed under reducedpressure. Water (500 mL) was added to the oily residue, followed by extraction with CH2Cl2 (200 mL,2x100 mL). The organic phase was washed with water (500 mL), sat. NaHCO3 (500 mL), and brine(500 mL), and dried over Na2SO4. The solvent was removed under reduced pressure and the residuewas filtered through silica gel (1:1 CH2Cl2:hexane). This gave 3b as a yellow oil, which was useddirectly in the next reaction without further purification. Yield: 23.7 g, 88.6 mmol, 89%.
References:
Hylland, Knut T.;Oien-Odegaard, Sigurd;Lillerud, Karl Petter;Tilset, Mats [Synlett,2015,vol. 26,# 11,art. no. ST-2015-B0194-L,p. 1480 - 1485] Location in patent:supporting information