Ethyl 4-chloro-5-Methoxypicolinate synthesis
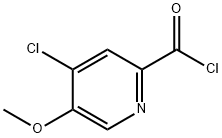
- Product Name:Ethyl 4-chloro-5-Methoxypicolinate
- CAS Number:40473-02-7
- Molecular formula:C9H10ClNO3
- Molecular Weight:215.63
Yield:40473-02-7 37%
Reaction Conditions:
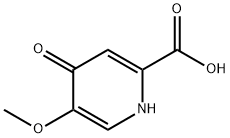
Stage #1: 5-methoxy-4-oxo-1,4-dihydropyridine-2-carboxylic acidwith thionyl chloride at 100; for 18 h;
Stage #2: ethanol for 0.333333 h;Reflux;
Steps:
58.1 Step 1. Synthesis of ethyl 4-chloro-5-methoxypyridine-2-carboxylate (C59).
A mixture of 5-methoxy-4-oxo-1 ,4-dihydropyridine-2-carboxylic acid (30.0 g, 177 mmol) and thionyl chloride (250 mL) was stirred at 100 °C for 18 hours, whereupon the reaction mixture was concentrated in vacuo. The residue was dissolved in anhydrousethanol (200 mL); the resulting solution was heated at reflux for 20 minutes and then cooled to 20 °C. After the mixture had been neutralized by addition of anhydrous sodium carbonate, it was filtered. The filtrate was cooled in an ice-ethanol bath, and stirred for 30 minutes; the precipitate was collected via filtration to afford the product as an off-white solid. The resulting filtrate was concentrated to a smaller volume under reduced pressure and cooled in an ice-ethanol bath. The precipitate was collected via filtration, providing additional product. Combined yield: 14.2 g, 65.8 mmol, 37%. LCMSm/z215.8 [M+H]. 1H NMR (400 MHz, DMSO-d6) ? 8.58 (5, 1H), 8.09 (5, 1H), 4.32 (q, J=7.l Hz, 2H), 4.08 (5, 3H), 1.32 (t, J=7.1 Hz, 3H).
References:
WO2016/9297,2016,A1 Location in patent:Page/Page column 140; 141