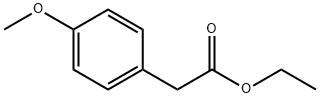
ETHYL 4-METHOXYPHENYLACETATE synthesis
- Product Name:ETHYL 4-METHOXYPHENYLACETATE
- CAS Number:14062-18-1
- Molecular formula:C11H14O3
- Molecular Weight:194.23
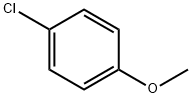
623-12-1
286 suppliers
$10.00/25g

6148-64-7
398 suppliers
$5.00/10g

14062-18-1
113 suppliers
$10.00/1g
Yield:14062-18-1 88%
Reaction Conditions:
with dmap;bis(η3-allyl-μ-chloropalladium(II));ruphos in 1,3,5-trimethyl-benzene at 140; for 20 h;Inert atmosphere;
Steps:
4.2. Pd-catalyzed decarboxylative cross-couplings of potassium malonate monoesters with aryl halides
General procedure: after standard cycles of evacuation and back-filling with dry and pure nitrogen, an oven-dried Schlenk tube equipped with a magnetic stirring bar was charged with Pd source (see Table 1, Table 2, Table 3 and Table 4), ligand (see Table 1, Table 2, Table 3 and Table 4), N,N-dimethylpyridin-4-amine (DMAP, see Table 1, Table 2, Table 3 and Table 4), and ethyl potassium malonate (see Table 1, Table 2, Table 3 and Table 4). The tube was evacuated and backfilled with argon (this procedure was repeated three times). Under a counter flow of argon, aryl halide (see Table 1, Table 2, Table 3 and Table 4) and solvent (see Table 1, Table 2, Table 3 and Table 4) were added by syringe. The tube was sealed and stirred at room temperature for 10 min. Then the tube was connected to the Schlenk line, which was full of argon, stirred in a preheated oil bath (140-150 °C) for the appointed time (20-25 h). Upon completion of the reaction, the mixture was cooled to room temperature and diluted with diethyl ether, and the yields were determined by gas chromatography using 1,3-dimethoxybenzene as the internal standard.
References:
Feng, Yi-Si;Wu, Wei;Xu, Zhong-Qiu;Li, Yan;Li, Ming;Xu, Hua-Jian [Tetrahedron,2012,vol. 68,# 9,p. 2113 - 2120] Location in patent:experimental part
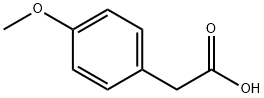
104-01-8
542 suppliers
$6.00/10g

64-17-5
746 suppliers
$10.00/50g

14062-18-1
113 suppliers
$10.00/1g
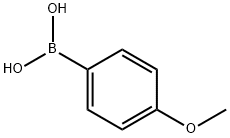
5720-07-0
470 suppliers
$6.00/5g

105-36-2
356 suppliers
$5.00/5 g

14062-18-1
113 suppliers
$10.00/1g

104-93-8
400 suppliers
$6.00/25g

64-17-5
746 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry

14062-18-1
113 suppliers
$10.00/1g
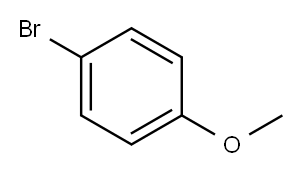
104-92-7
429 suppliers
$10.00/10g

6148-64-7
398 suppliers
$5.00/10g

14062-18-1
113 suppliers
$10.00/1g