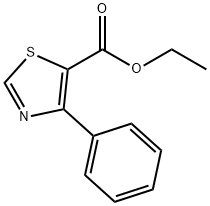
ethyl 4-phenylthiazole-5-carboxylate synthesis
- Product Name:ethyl 4-phenylthiazole-5-carboxylate
- CAS Number:99822-80-7
- Molecular formula:C12H11NO2S
- Molecular Weight:233.29

64399-23-1
101 suppliers
$26.00/250mg

99822-80-7
24 suppliers
$74.00/100mg
Yield:99822-80-7 30%
Reaction Conditions:
Stage #1: ethyl 4-phenyl-2-aminothiazole-5-carboxylatewith sulfuric acid;sodium nitrite in water at 0;
Stage #2: with hypophosphorous acid in water at 0 - 5; for 1 h;
Steps:
17 4.1.17. Ethyl 4-phenyl-1,3-thiazole-5-carboxylate (22)
4.1.17
Ethyl 4-phenyl-1,3-thiazole-5-carboxylate (22)
To a mixture of ethyl 2-amino-4-phenyl-1,3-thiazole-5-carboxylate
14
(21 6.00 g, 24.2 mmol) and 50% aqueous H2SO4 (80 mL), NaNO2 (2.0 g, 29.0 mmol) in H2O (10 mL) was added dropwise below 0 °C. Subsequently, 50% aqueous H3PO2 (35.1 g, 266 mmol) was added to the mixture below 0 °C.
The mixture was stirred for 1 h below 5 °C and then diluted with H2O (100 mL) and adjusted to pH 9-10 with 10 M NaOH.
The mixture was extracted with AcOEt, and the organic layer was dried and concentrated in vacuo.
The residue was chromatographed on silica gel with elution using hexane/AcOEt (97:3 to 85:15) to produce the desired compound 22 (1.68 g, 30%) as an orange oil. 1H NMR (DMSO-d6) δ 1.21 (3H, t, J = 7.2 Hz), 4.23 (2H, q, J = 7.2 Hz), 7.43-7.47 (3H, m), 7.70-7.75 (2H, m), 9.33 (1H, s); FAB MS m/e [M+H]+ 234.
References:
Nagashima, Shinya;Matsushima, Yuji;Hamaguchi, Hisao;Nagata, Hiroshi;Kontani, Toru;Moritomo, Ayako;Koshika, Tadatsura;Takeuchi, Makoto [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 13,p. 3478 - 3487]

115-08-2
51 suppliers
inquiry
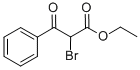
55919-47-6
56 suppliers
$45.00/50mg

99822-80-7
24 suppliers
$74.00/100mg