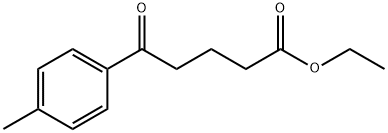
ETHYL 5-(4-METHYLPHENYL)-5-OXOVALERATE synthesis
- Product Name:ETHYL 5-(4-METHYLPHENYL)-5-OXOVALERATE
- CAS Number:42482-94-0
- Molecular formula:C14H18O3
- Molecular Weight:234.29
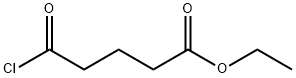
5205-39-0
86 suppliers
$31.00/5g
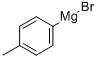
4294-57-9
124 suppliers
$50.00/10ml

42482-94-0
10 suppliers
$236.81/1gm:
Yield:-
Reaction Conditions:
with copper(I) bromide;lithium bromide in tetrahydrofuran at 0; for 18 h;Inert atmosphere;
Steps:
5a
To a solution of lithium bromide (24 g, 277.06 mmol) in 500 ml of dry tetrahydrofurane, stirred under nitrogen atmosphere, copper(I) bromide (19,87 g, 138,52 mmol) was added. The reaction mixture was stirred at room temperature until a solution was obtained. Then, the reaction mixture was cooled to 0°C and a 0.5M solution of commercially available 4-tolyl magnesium bromide in THF (277.05 ml, 138,52 mmol) was added. Then, commercially available 4-chlorocarbonyl-butyric acid ethyl ester (19 g, 115,44 mmol) was added and the reaction mixture was stirred at 0°C for 18h.500 ml of a saturated aqueous ammonium chloride solution was added and the reaction mixture was extracted twice with dichloro methane. The organic phase was washed with a saturated aqueous sodium bicarbonate solution, dried over sodium sulfate and concentrated under vacuum. The crude product (20 g) was used in the next step without any purification.
References:
WO2011/73155,2011,A1 Location in patent:Page/Page column 90

64-17-5
729 suppliers
$10.00/50g
833-85-2
36 suppliers
$104.00/1g

42482-94-0
10 suppliers
$236.81/1gm:
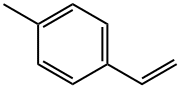
622-97-9
136 suppliers
$10.00/5mL

42482-94-0
10 suppliers
$236.81/1gm:
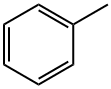
108-88-3
7 suppliers
$14.00/100ml

42482-94-0
10 suppliers
$236.81/1gm:

108-55-4
441 suppliers
$6.00/25g

42482-94-0
10 suppliers
$236.81/1gm: