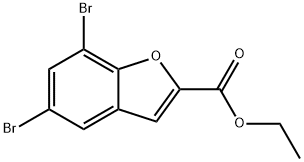
Ethyl 5,7-Dibromo-1-Benzofuran-2-Carboxylate synthesis
- Product Name:Ethyl 5,7-Dibromo-1-Benzofuran-2-Carboxylate
- CAS Number:91182-70-6
- Molecular formula:C11H8Br2O3
- Molecular Weight:347.99
Yield:91182-70-6 240 g
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100;
Steps:
Step A: Synthesis of Int A-1
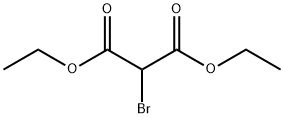
To a solution of 3,5-dibromo-2-hydroxybenzaldehyde (400 g, 1.44 mol) and ethyl 2- bromoacetate (360 g, 2.16 mol) in DMF (1800 mL) was added anhydrous potassium carbonate (590 g, 4.29 mol) in one portion at room temperature. The mixture was heated at 100°C and magnetically stirred at this temperature overnight. The mixture was cooled to room temperature and the solids were removed by filtration. The filter cake was washed with EtOAc (500 mL c 3) and the filtrate was concentrated under reduce pressure with rotary-evaporator to remove EtOAc. The residue was poured into ice water (w/w = 1/1 , 4 L) whereby a yellow solid formed. The solid was collected by filtration and washed with MeOH (200 mL) three times. The solid was dried under reduced pressure to give 240 g of compound Int A-1 which was used directly in the next step. Rf = 0.5 (Petroleum Ether : EtOAc =20: 1)
References:
WO2019/152911,2019,A1 Location in patent:Page/Page column 50