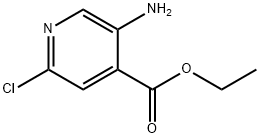
Ethyl 5-amino-2-chloropyridine-4-carboxylate synthesis
- Product Name:Ethyl 5-amino-2-chloropyridine-4-carboxylate
- CAS Number:862314-10-1
- Molecular formula:C8H9ClN2O2
- Molecular Weight:200.62
14208-83-4
133 suppliers
$11.00/1g

862314-10-1
10 suppliers
inquiry
Yield:862314-10-1 41%
Reaction Conditions:
with N-chloro-succinimide in N,N-dimethyl-formamide at 20 - 50; for 18 h;Inert atmosphere;
Steps:
56.56.A 56A): Ethyl 5-amino-2-chloroisonicotinate
To a solution of ethyl 3-aminoisonicotinate (4.0 g, 24.07 mmol) in DMF (25 mL) was added NCS (3.54 g, 26.5 mmol) at room temperature. The reaction mixture was then heated at 50° C. under nitrogen for 18 h. The reaction mixture was cooled to room temperature and treated with saturated aqueous NaHCO3 solution and ethyl acetate. The organic layer was separated and washed with brine, dried (MgSO4) and filtered. The filtrate was concentrated in vacuo. The residue was dissolved in DCM and purified by silica gel flash chromatography, eluting with 30% ethyl acetate in hexane to give the desired product as a light yellow solid (2.0 g, 41%); HPLC: RT=0.84 min (H2O/ACN with 0.05% TFA, Waters Acquity SDS BEH C18, 2.1×50 mm, 1.7-μm particles, gradient=1.8 min, wavelength=220 nm); MS (ES): m/z=200.9 [M+H]+; 1H NMR (400 MHz, CDCl3) δ ppm 7.98 (d, J=0.4 Hz, 1H), 7.66 (d, J=0.4 Hz, 1H), 4.38 (q, J=7.3 Hz, 2H), 1.42 (t, J=7.2 Hz, 3H).
References:
US2016/176871,2016,A1 Location in patent:Paragraph 0495-0496