
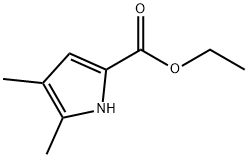
Ethyl 5-forMyl-4-Methyl-1H-pyrrole-2-carboxylate synthesis
- Product Name:Ethyl 5-forMyl-4-Methyl-1H-pyrrole-2-carboxylate
- CAS Number:26018-26-8
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
Yield:26018-26-8 99%
Reaction Conditions:
Stage #1: 4-Methyl-1H-pyrrole-2-carboxylic acid ethyl ester;Vilsmeier reagent in 1,2-dichloro-ethane at 20;
Stage #2: with sodium hydroxide;water pH=10;
Steps:
1
To a solution of (chloro-methylen)-dimethyl-ammonium-chloride (1.64 g, 12.8 mmoles) in dry DCE (6 mL) was added dropwise under argon atmosphere a solution of 4-methyl- lH-pyrrole-2-carboxylic acid ethyl ester (1.31 g, 8.6 mmoles) in DCE (5 mL). The reaction mixture was heated slightly to dissolve all solid particules and stirred at room temperature overnight. TLC indicated that there was no starting material left. The pH of the reaction mixture was adjusted to 10 by addition of NaOH (2 N). The crude mixture was extracted with CH2CI2 (3x10 mL). The combined organic phases were washed once with H2O, once with brine, dried over Mg504 and filtered. After removal of the solvent, 1.49 g of the title compound (99% yield) were obtained as a pale red/orange solid and used in the next step without further purification. 'H NMR (DMSO-d6) 8 12.6 (br, lH), 9.80 (s, lH), 6.70 (s, lH), 4.27 (q, 2H), 2.30 (s, 3H), 1.30 (t, 3H).
References:
WO2005/58309,2005,A1 Location in patent:Page/Page column 49-50
2199-45-3
45 suppliers
$90.00/100mg

26018-26-8
5 suppliers
inquiry

40611-85-6
93 suppliers
$50.00/250mg

26018-26-8
5 suppliers
inquiry
1414887-10-7
1 suppliers
inquiry

26018-26-8
5 suppliers
inquiry
