
Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate synthesis
- Product Name:Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate
- CAS Number:1101120-55-1
- Molecular formula:C10H9IN2O2
- Molecular Weight:316.1
![ethyl 5-((tert-butoxycarbonyl)aMino)pyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/GIF/1101120-33-5.gif)
1101120-33-5
45 suppliers
$45.00/10mg
![Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20180629/GIF/1101120-55-1.gif)
1101120-55-1
8 suppliers
inquiry
Yield:1101120-55-1 58%
Reaction Conditions:
Stage #1: 5-[(tert-butoxycarbonyl)amino]pyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl esterwith trifluoroacetic acid in dichloromethane at 20; for 3 h;Cooling with ice;
Stage #2: with hydrogenchloride;sulfuric acid;sodium nitrite in water; for 0.25 h;Cooling with ice;
Stage #3: with potassium iodide in water at 20; for 0.5 h;
Steps:
8.2 Step 2:Preparation of 5-iodopyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester
The compound 5-((tert-butoxycarbonyl)amino)pyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (3.3g, 10.82mmol) was dissolved in DCM (50mL),It was cooled and stirred under an ice water bath, and TFA (15 mL) was added dropwise thereto.After the addition is complete, remove the ice-water bath and return to room temperature and stir for 3h.After TLC showed that the reaction was complete, the reaction solution was concentrated to dryness to obtain a brown oil.The oil was dissolved in concentrated hydrochloric acid (30 mL),Concentrated sulfuric acid (3 mL) was added dropwise to it under ice bath. NaNO2 (821mg, 11.90mmol)Dissolve in water (15mL), slowly drip into the reaction system,Maintain the ice-water bath and stir for 15 min. Add a small amount of urea (50mg) to the reaction system,Continue to stir for 15 minutes. KI (2.89g, 17.31mmol)The solid was added to the reaction system at one time, and the reaction solution was returned to room temperature and stirred for 30 minutes.The reaction solution was adjusted to pH=3 with solid NaOH, and extracted twice with DCM.The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to dryness.The residue was purified by column chromatography (PE/EA=10/17/1) to obtain the target compound (2.0g, yield 58%) as a yellow solid.
References:
CN112110938,2020,A Location in patent:Paragraph 0347-0348; 0352-0355
![Ethyl 5-AMinoopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/GIF/1101120-35-7.gif)
1101120-35-7
49 suppliers
$45.00/50mg
![Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20180629/GIF/1101120-55-1.gif)
1101120-55-1
8 suppliers
inquiry
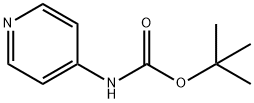
98400-69-2
171 suppliers
$6.00/1g
![Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20180629/GIF/1101120-55-1.gif)
1101120-55-1
8 suppliers
inquiry

623-47-2
334 suppliers
$10.00/1g
![Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20180629/GIF/1101120-55-1.gif)
1101120-55-1
8 suppliers
inquiry
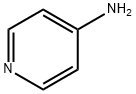
504-24-5
469 suppliers
$10.00/1g
![Ethyl 5-iodopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20180629/GIF/1101120-55-1.gif)
1101120-55-1
8 suppliers
inquiry