
Ethyl 5-methoxybenzo[b]thiophene-2-carboxylate synthesis
- Product Name:Ethyl 5-methoxybenzo[b]thiophene-2-carboxylate
- CAS Number:40862-88-2
- Molecular formula:C12H12O3S
- Molecular Weight:236.29
105728-90-3
169 suppliers
$12.00/1g

623-51-8
233 suppliers
$7.00/25g
![Ethyl 5-methoxybenzo[b]thiophene-2-carboxylate](/CAS/20200331/GIF/40862-88-2.gif)
40862-88-2
14 suppliers
inquiry
Yield:40862-88-2 66%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 80; for 1 h;
Steps:
17.A
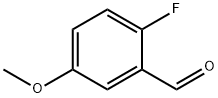
A solution of 2-fluoro-5-methoxy-benzaldehyde (4.35 g,28. 2 mmol) in DMF (30mL) is added mercapto-acetic acid ethyl ester (3.71mL, 33.9 mmol) andK2CO3 (7.86 g, 57.0mmol). The resulting suspension is stirred at80 C for 60 min and quenched with water (300 mL). The mixture is extracted with EtOAc (2 x 200 mL), and the organic layer is dried overNa2SO4, concentrated, and purified by silica gel column chromatography(10% EtOAc/Hex), to give the title compound as an oil (4.40 g, 66%). H-NMR (ppm, CDCl3),No. : 7.97 (1 H, s), 7.70 (1 H, d,J=8. 8 Hz), 7.27 (1 H, d,J=2. 6 Hz), 7.09 (1 H, dd,J=2. 6,8.8 Hz), 4.41 (2 H, q,J=7. 0 Hz), 3.88 (3 H, s), 1.42 (3 H, t, J=7. 0 Hz).
References:
WO2005/51940,2005,A2 Location in patent:Page/Page column 132