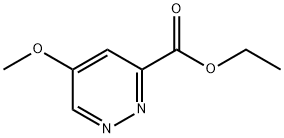
ethyl 5-methoxypyridazine-3-carboxylate synthesis
- Product Name:ethyl 5-methoxypyridazine-3-carboxylate
- CAS Number:627525-71-7
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18

64-17-5
706 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry
123696-02-6
132 suppliers
$17.00/250mg

627525-71-7
7 suppliers
inquiry
Yield:627525-71-7 80%
Reaction Conditions:
with triethylamine;[1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) in ethanol at 120; under 30003 Torr; for 5 h;
Steps:
84; 1 EXAMPLE 84; 5-Methoxy-pyridazine-3-carboxylic Acid Ethyl Ester
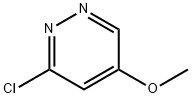
3-Chloro-5-methoxy-pyridazine (Bryant, R. D.; Kunng, F.-A.; South, M. S. J. Heterocycl. Chem. (1995), 32(5), 1473-6.) (21 g, 0.145 mol), bis(triphenylphosphine)palladium dichloride (10.2 g, 14.5 mmol) and triethylamine (30.5 ml, 0.218 mol) in ethanol (420 ml) were heated at 120° C. under a 40 bar pressure of carbon monoxide for 5 hours. The reaction mixture was cooled, filtered and concentrated in vacuo. The residue was taken in CH2Cl2 (200 ml), washed with H2O (twice), dried over Na2SO4 and concentrated in vacuo. The crude solid was stirred in Et2O and filtered to provide 22.1 g of a light brown solid which was chromatographed over silica gel (hexane - ethylacetate 99 : 1 to 50 : 50) to provide 5-methoxy-pyridazine-3-carboxylic acid ethyl ester (21.1 g, 80%) as a pale yellow solid, MS: m/e=183.2 (M+H+).
References:
US2003/229096,2003,A1 Location in patent:Page 16; 6