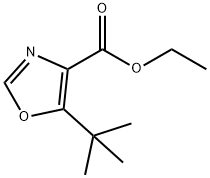
Ethyl 5-tert-butyl-1,3-oxazole-4-carboxylate synthesis
- Product Name:Ethyl 5-tert-butyl-1,3-oxazole-4-carboxylate
- CAS Number:714273-89-9
- Molecular formula:C10H15NO3
- Molecular Weight:197.23
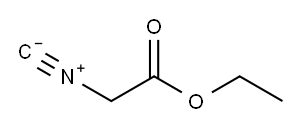
2999-46-4
217 suppliers
$13.43/1gm:
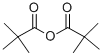
1538-75-6
202 suppliers
$17.67/10gm:

714273-89-9
27 suppliers
$65.00/100mg
Yield:714273-89-9 99%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in tetrahydrofuran at 4 - 20;
Steps:
3
Ethyl 5-(tert-butyl)oxazole-4-carboxylate (2); [0204] According to the report by Suzuki et al. (JOC, 38, 3571-3575 (1973)), to a stirring solution of ethyl isocyanoacetate 1 (25 g, 221 mmol, caution: bad smell, it should be treated in a draft chamber, Wako Pure Chemical, Osaka, Japan, Cat. No. 055-06672, Rf 0.70 [CHCl3:MeOH = 10:0.5]) in anhydrous THF (200 mL, Kanto Chemical, Tokyo, Japan, Cat. No. 40993-05) was added DBU (34.3 mL, 243 mmol, Nacalai Tesque, Kyoto, Japan, Cat. No. 11117-05) and pivalic anhydride (49.3 mL, 243 mmol, Wako Pure Chemical, Osaka, Japan, Cat. No. 168-19661) at 4 °C. After 10 min, the ice-bath was removed and the mixture was stirred overnight at room temperature. start ng mate a reaction mixture[0205] Then, the solvent of the obtained dark brown reaction mixture was removed by evaporation in vacuo. AcOEt (200 mL) was added to the obtained residue, then this mixture was washed with 10% Na2C03 (x 3), 10% citric acid (x 3) and saturated NaCl (x 3), and dried over anhydrous Na2S04, and the solvent was evaporated in vacuo. The residual oil was dissolved in CHC13 (20 mL) and applied to silica-gel column chromatography (6 x 30 cm, Merck 107734 silica gel 60, 70-230 mesh, prepared with Hexane: AcOEt = 20:1) and eluted with Hexane: AcOEt (20:1 to 4:1, the desired 2 is eluted at 8:1), to give an pale yellow oil of 2 (66.4 g, 99%). Rf 0.68 (CHCl3:MeOH = 10:0.5), 1H NMR (300MHz CDC13) δ 7.70 (s, 1H), 4.39 (q, J = 7.2 Hz, 2H), 1.46 (s, 9H), 1.41 (t, J = 7.2 Hz, 3H); HRMS(EI): m/z 197.1050 (M+) (Calcd for Ci0Hi5NO3: 197.1052).
References:
WO2011/84962,2011,A1 Location in patent:Page/Page column 44-45

2999-46-4
217 suppliers
$13.43/1gm:
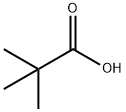
75-98-9
411 suppliers
$10.00/10g

714273-89-9
27 suppliers
$65.00/100mg

2999-46-4
217 suppliers
$13.43/1gm:

714273-89-9
27 suppliers
$65.00/100mg
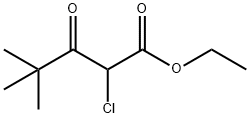
29509-07-7
2 suppliers
inquiry

60100-09-6
0 suppliers
inquiry
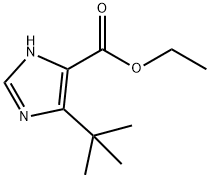
51721-21-2
26 suppliers
$486.54/1g

714273-89-9
27 suppliers
$65.00/100mg
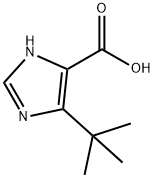
714273-88-8
14 suppliers
$356.00/500mg
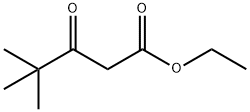
17094-34-7
106 suppliers
$21.00/5mL

714273-89-9
27 suppliers
$65.00/100mg

714273-88-8
14 suppliers
$356.00/500mg