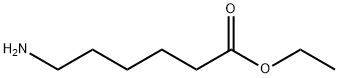
ETHYL 6-AMINOHEXANOATE synthesis
- Product Name:ETHYL 6-AMINOHEXANOATE
- CAS Number:371-34-6
- Molecular formula:C8H17NO2
- Molecular Weight:159.23
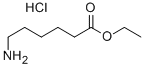
3633-17-8
60 suppliers
$65.00/1g

371-34-6
20 suppliers
$59.89/250mg
Yield:371-34-6 98.9%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 12.5 h;
Steps:
1.2 2) Preparation of Ethyl 6-Aminohexanoate
After 340.0 g (1.74 mol) of the ethyl 6-aminohexanoate hydrochloride prepared in 1) was put in a 2 L round-bottom flask and 700 ml of dichloromethane was added thereto, the temperature was set at 0° C. Thereafter, a reaction was performed for 12 hours by slowly increasing the temperature to room temperature while 605.86 ml (4.34 mol) of triethylamine was slowly added for 30 minutes, and the reaction was then terminated. After 500 ml of water was added to extract an organic layer, sodium sulfate was added to the organic layer, filtered, and then concentrated to obtain 273.7 g (yield: 98.9%) of ethyl 6-aminohexanoate. 1H nuclear magnetic resonance spectroscopic data of the ethyl 6-aminohexanoate are as follows. (0182) 1H-NMR (500 MHz, CDCl3) δ 4.13-4.09 (q, 2H), 2.69-2.67 (t, 2H), 2.30-2.27 (t, 2H), 1.60 (m, 2H), 1.48-1.42 (m, 2H), 1.37-1.31 (m, 2H), 1.25-1.23 (t, 2H), 1.19 (s, 3H).
References:
US2020/123287,2020,A1 Location in patent:Paragraph 0180-0182
![Hexanenitrile, 6-[(3,4,5,6-tetrahydro-2H-azepin-7-yl)amino]-](/CAS/20210305/GIF/185350-62-3.gif)
185350-62-3
0 suppliers
inquiry

2432-74-8
74 suppliers
inquiry
105-60-2
545 suppliers
$5.00/25g

371-34-6
20 suppliers
$59.89/250mg
![Hexanenitrile, 6-[(3,4,5,6-tetrahydro-2H-azepin-7-yl)amino]-](/CAS/20210305/GIF/185350-62-3.gif)
185350-62-3
0 suppliers
inquiry

105-60-2
545 suppliers
$5.00/25g

2432-74-8
74 suppliers
inquiry

371-34-6
20 suppliers
$59.89/250mg

105-60-2
545 suppliers
$5.00/25g

64-17-5
744 suppliers
$10.00/50g

371-34-6
20 suppliers
$59.89/250mg
60-32-2
605 suppliers
$5.00/10g