
ETHYL 6-CHLORO-1-BENZOTHIOPHENE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 6-CHLORO-1-BENZOTHIOPHENE-2-CARBOXYLATE
- CAS Number:105191-53-5
- Molecular formula:C11H9ClO2S
- Molecular Weight:240.71
61072-56-8
290 suppliers
$14.00/5g

623-51-8
236 suppliers
$7.00/25g

105191-53-5
24 suppliers
$489.00/5g
Yield:105191-53-5 60%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 70; for 16 h;
Steps:
B.9.1 Step-1: Synthesis of ethyl 6-chlorobenzothiophene-2-carboxylate Xl-9a:
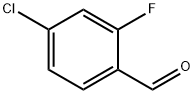
To a solution of 4- chloro--2--fluorobenzaldehyde (10.0 g, 63.0 mmol) in DMF (100 ml) wasadded ethyl thioglycolate (11.3 g, 94.6 mmol) followed by addition of K2C03 (26.1 g, 189mmol). The reaction mixture was heated at 70°C for 16h. Progress of the reaction wasmonitored by TLC. After completion, the reaction mixture was diluted with H20 (300 ml)10 and extracted with EtOAc (2 x 300 ml). The organic layer was separated, dried overanhydrous Na2S04 and concentrated under vacuum to afford ethyl 6-chlorobenzothiophene-2-carboxylate Xl-9a (9.1 0 g) as an off-white solid.This compound was used as such for the next reaction without further purification.Yield: 60%15 1H NMR (400 MHz, DMSO-d5) o 1.33 (t, J=7.09 Hz, 3H) 4.35 (q, J=7.01 Hz, 2H) 7.51 (dd,J=8.56, 1.71 Hz, 1 H) 8.04 (d, J=8.80 Hz, 1 H) 8.20 (s, 1 H) 8.25 (d, J=0.98 Hz, 1 H).
References:
WO2018/122232,2018,A1 Location in patent:Page/Page column 245

64-17-5
730 suppliers
$10.00/50g
![Benzo[b]thiophene-2-carbonyl chloride, 6-chloro-](/CAS/20210305/GIF/105191-51-3.gif)
105191-51-3
1 suppliers
inquiry

105191-53-5
24 suppliers
$489.00/5g
26018-73-5
76 suppliers
$19.00/100mg

105191-53-5
24 suppliers
$489.00/5g