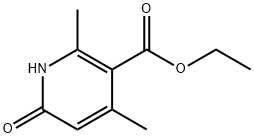
ethyl 6-hydroxy-2,4-diMethylnicotinate synthesis
- Product Name:ethyl 6-hydroxy-2,4-diMethylnicotinate
- CAS Number:36853-14-2
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
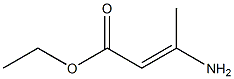
41867-20-3
11 suppliers
inquiry

36853-14-2
16 suppliers
inquiry
Yield: 29%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;toluene at 115;
Steps:
1
(E) -Ethyl 3-aminobut-2-enoate (2.53 g, 19.6 mmol) in toluene (15 mL) was charged with 4N HCl (10 mL, 40 mmol) in dioxane. The mixture was stirred at 115°C overnight. The solution was cooled and the solid was filtered off washing with toluene. The filtrate was concentrated and the residue was purified by silica gel column chromatography (5% MeOH/EtOAc) to
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED WO2008/11131, 2008, A2 Location in patent:Page/Page column 251-252

5977-14-0
170 suppliers
$35.70/100g
141-97-9
783 suppliers
$5.00/25g

36853-14-2
16 suppliers
inquiry
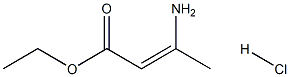
89095-42-1
0 suppliers
inquiry

36853-14-2
16 suppliers
inquiry
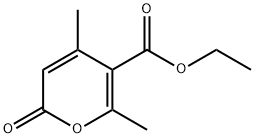
3385-34-0
91 suppliers
$100.00/25g
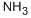
7664-41-7
491 suppliers
$15.00/100ml

36853-14-2
16 suppliers
inquiry