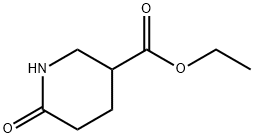
Ethyl 6-oxopiperidine-3-carboxylate synthesis
- Product Name:Ethyl 6-oxopiperidine-3-carboxylate
- CAS Number:146059-76-9
- Molecular formula:C8H13NO3
- Molecular Weight:171.19

64-17-5
706 suppliers
$10.00/50g
22540-50-7
75 suppliers
$50.00/10mg

146059-76-9
22 suppliers
$45.00/25mg
Yield:146059-76-9 35.2%
Reaction Conditions:
with thionyl chloride at 20;
Steps:
A2-2.3 Step 3:
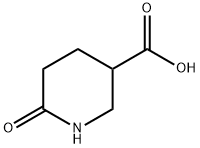
To a mixture of 6-oxopiperidine-3-carboxylic acid (500 mg, 3.49 mmol, 1.0 eq) in EtOH (3 mL) was added thionyl chloride (5.24 mmol, 0.38 mL, 1.5 eq) dropwise. The mixture was stirred at room temperature overnight. The mixture was concentrated in vacuum to afford the crude product. After washed with EtOAc (2 mL), the solid was collected. The solid was dried in vacuum to afford the desired product (480 mg, 72.2%). LCMS: M+H=172.2, Ref. time=0.514.1H NMR (400 MHz, DMSO) δ 10.28(s, 1H), 4.13-4.07(m, 2H), 3.34-3.23(m, 2H), 2.83-2.77(m, 1H), 2.27-2.12(m, 2H), 2.02-1.95(m, 1H), 1.88-1.79(m, 1H),1.21-1.17(t, J=6.8Hz, J=14Hz, 3H)
References:
WO2022/236272,2022,A2 Location in patent:Paragraph 00469; 00574; 00576

22540-50-7
75 suppliers
$50.00/10mg

146059-76-9
22 suppliers
$45.00/25mg