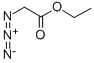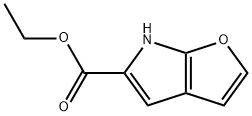
ETHYL 6H-FURO[2,3-B]PYRROLE-5-CARBOXYLATE synthesis
- Product Name:ETHYL 6H-FURO[2,3-B]PYRROLE-5-CARBOXYLATE
- CAS Number:58326-31-1
- Molecular formula:C9H9NO3
- Molecular Weight:179.17
Yield:58326-31-1 33%
Reaction Conditions:
with sodium ethanolate in 5,5-dimethyl-1,3-cyclohexadiene;ethanol at 0; for 6 h;Reflux;
Steps:
5.2 Step 2: Synthesis of 6H-furo[2,3-b]pyrrole-5-carboxylic acid ethyl ester
Under nitrogen protection, sodium scrap (7.64 g, 0.33 mol, 4.0 eq) was added to absolute ethanol (400 mL), stirred until the reaction was complete, and the temperature was lowered to 0 °C.A solution of 2-azidoacetic acid ethyl ester (43 g, 0.33 mol, 4.0 eq) and furan-3-carbaldehyde (8 g, 0.083 mol, 1.0 eq) in ethanol (40 mL) was then evaporated.The reaction was carried out at 0 ° C for 1 h, and then raised to room temperature for 1 h. The reaction was completely detected by TLC, and the reaction was quenched by dropwise addition of saturated aqueous ammonium chloride (200 mL).The mixture was extracted with methyl tert-butyl ether (300 mL × 3), and the organic phase was combined, washed with brine (300 mL × 3), dried and concentrated. The obtained crude product was added to xylene (100 mL), and the mixture was stirred and refluxed for 4h.The reaction was complete and concentrated by TLC and LC-MS.The crude product was purified by silica gel column chromatography to yield yellow solidThe product was obtained (5.14 g, yield: 33%).
References:
CN110194770,2019,A Location in patent:Paragraph 0463; 0464; 0468; 0469; 0470
488-93-7
380 suppliers
$5.00/250mg
![ETHYL 6H-FURO[2,3-B]PYRROLE-5-CARBOXYLATE](/CAS/20180601/GIF/58326-31-1.gif)
58326-31-1
9 suppliers
inquiry
4412-91-3
193 suppliers
$14.00/1g
![ETHYL 6H-FURO[2,3-B]PYRROLE-5-CARBOXYLATE](/CAS/20180601/GIF/58326-31-1.gif)
58326-31-1
9 suppliers
inquiry
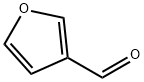
498-60-2
259 suppliers
$16.00/1g
![ETHYL 6H-FURO[2,3-B]PYRROLE-5-CARBOXYLATE](/CAS/20180601/GIF/58326-31-1.gif)
58326-31-1
9 suppliers
inquiry