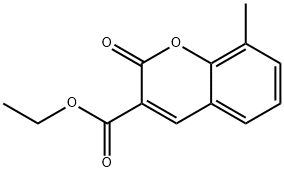
Ethyl 8-methyl-2-oxo-2H-chromene-3-carboxylate synthesis
- Product Name:Ethyl 8-methyl-2-oxo-2H-chromene-3-carboxylate
- CAS Number:17349-63-2
- Molecular formula:C13H12O4
- Molecular Weight:232.23
Yield:17349-63-2 78%
Reaction Conditions:
with piperidine;acetic acid in ethanol; for 0.0833333 h;Sonication;
Steps:
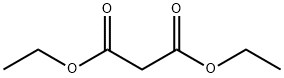
3-Ethoxycarbonylcoumarins 2-7; General Ultrasonic Procedure
General procedure: The 3-ethoxycarbonylcoumarin derivatives were prepared from a 1:1.1 mol ratio of the appropriate salicylaldehyde (500 mg, 4.1 mmol) and diethyl malonate (722 mg, 4.51 mmol) in absolute EtOH (2 mL). To this mixture, piperidine (34.5 mg, 0.4 mmol) and a catalytic amount of glacial AcOH were added. Ultrasonic irradiation was performed (frequency = 20 kHz, amplitude = 90% of the maximum power output) without a pulse for 5-30 min. After this period of time, hot H2O (60 °C) (3 mL) was added, and after cooling the mixture at r.t., it was stored overnight in a refrigerator. The product was collected by filtration and washed with a solution of EtOH (1 mL) and distilled H2O (2 mL). Finally, the product was washed with distilled H2O (20 mL).
References:
Da Silveira Pinto, Ligia S.;De Souza, Marcus V. N. [Synthesis,2017,vol. 49,# 12,p. 2677 - 2682]