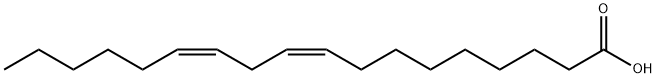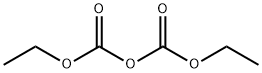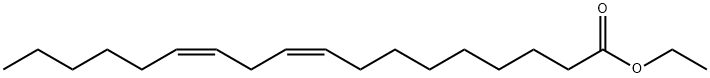
ETHYL LINOLEATE synthesis
- Product Name:ETHYL LINOLEATE
- CAS Number:544-35-4
- Molecular formula:C20H36O2
- Molecular Weight:308.5
Yield:544-35-4 91.07%
Reaction Conditions:
with 1-hexyl-3-methylimidazolium hydrogen sulphate in cyclohexane at 40; for 8 h;Reagent/catalyst;Temperature;
Steps:
1-6 Example 6
Ionic liquid microemulsion components and their dosage: hydrophilic ionic liquid [Hmim]HSO4 (purchased from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences) 4 parts by weight, 5 parts by weight cyclohexane, conventional surfactant TX-100 ( (Purchased from Guangzhou Jingke Chemical Glass Instrument Company) 24 parts by weight.Under the condition of 25, mix the surfactant TX-100 and the imidazole ionic liquid 1-hexyl-3-methylimidazole bisulfate evenly at a stirring speed of 700 rpm, stirring for 5 minutes to obtain a clear and transparent mixture. Then add cyclohexane and mix it uniformly at a stirring speed of 700 rpm and stir for 5 minutes to obtain a clear and transparent ionic liquid microemulsion with good high temperature stability.Take 7.7131 g of ionic liquid microemulsion, 5.6112 g of linoleic acid, and 5.5653 g of ethanol to stir and mix, and carry out the esterification reaction under stirring, wherein the stirring speed is 700 revolutions/min, the reaction temperature is 40° C., and the reaction time is 8 hours. During the reaction process, 1-hexyl-3-methylimidazole hydrogensulfate can promote the formation of ionic liquid microemulsions and increase the contact area of the catalyst 1-hexyl-3-methylimidazole hydrogensulfate with linoleic acid and ethanol. And it can form a hydrogen bond with the water generated by the esterification reaction, and transfer the water generated by the reaction to the inner core of the ionic liquid microemulsion during the esterification reaction to promote the forward movement of the esterification reaction.After the reaction is completed, the product is transferred to a rotary evaporator, the vacuum pump is turned on, and the product is rotary evaporated at 50° C. for 1 hour to obtain the product. The product was identified as ethyl linoleate by gas chromatography, and the yield of ethyl linoleate was 91.07%.
References:
CN111302935,2020,A Location in patent:Paragraph 0033-0062

64-17-5
715 suppliers
$10.00/50g
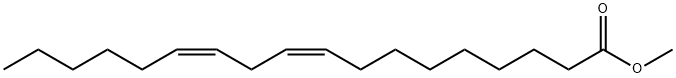
112-63-0
257 suppliers
$15.00/5g
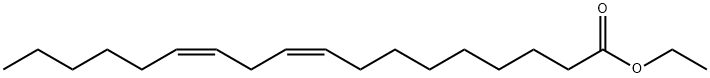
544-35-4
254 suppliers
$12.00/50mg

56318-83-3
0 suppliers
inquiry
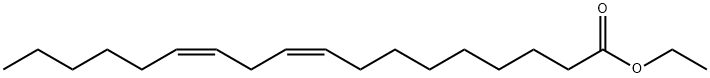
544-35-4
254 suppliers
$12.00/50mg

4286-55-9
300 suppliers
$15.00/10g
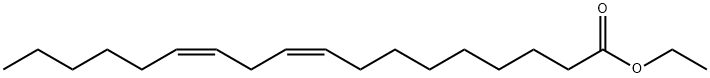
544-35-4
254 suppliers
$12.00/50mg