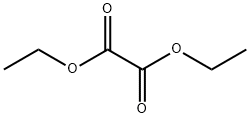
ethyl nicotinoylpyruvate synthesis
- Product Name:ethyl nicotinoylpyruvate
- CAS Number:92288-94-3
- Molecular formula:C11H11NO4
- Molecular Weight:221.21
Yield:92288-94-3 89%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil; for 0.25 h;Heating;Reflux;
Steps:
36.1
Step 1 Synthesis of 2,4-Dioxo-4-pyridin-3-yl-butyric acid ethyl ester To a stirred solution of NaH (60% w/w dispersion in oil) (991 mg, 24.8 mmol) in THF (25 mL), was added diethyl oxalate (2.41 g, 16.52 mmol) and the resulting mixture was heated to reflux. At reflux temperature, 1-pyridin-3-yl-ethanone (1.0 g, 8.26 mmol) was added and stirring was continued at reflux temperature for a further 15 minutes. The reaction mixture was then quenched with ice cold water, acidified with aqueous 2.4N HCl solution and extracted with ethyl acetate. The organic layer was dried over sodium sulfate and concentrated under reduced pressure to afford 1.62 g (89%) of 2,4-dioxo-4-pyridin-3-yl-butyric acid ethyl ester.
References:
US2010/160323,2010,A1 Location in patent:Page/Page column 35-36