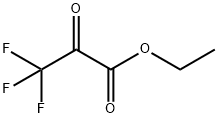
Ethyl trifluoropyruvate synthesis
- Product Name:Ethyl trifluoropyruvate
- CAS Number:13081-18-0
- Molecular formula:C5H5F3O3
- Molecular Weight:170.09
Yield:13081-18-0 96 %
Reaction Conditions:
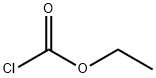
Stage #1: chloroformic acid ethyl esterwith n-butyllithium in hexane at -40 - -35;Inert atmosphere;Sealed tube;
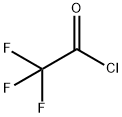
Stage #2: trifluoracetyl chloride at -10 - 25;
Steps:
3 Example 3 Preparation of ethyl trifluoropyruvate
A 500mL dry anhydrous three-necked glass reaction flask equipped with a stirring magnet and a thermometer in a low-temperature reaction bath.After the reaction bottle was purged with high-purity nitrogen gas (so that there was no oxygen in the bottle), one grinding port was connected to a nitrogen bag, and the other grinding port was sealed with a rubber stopper.Insert the syringe into the rubber stopper and use the syringe to add 65g of n-hexane and 21.7g (0.2mol) of ethyl chloroformate into the reaction bottle. After the addition is complete, start the stirring magnet and keep stirring continuously, cool down to -40-35°C and keep At this temperature, add 125mL of n-butyllithium n-hexane solution (the concentration of n-butyllithium in the n-hexane solution of n-butyllithium is 1.6mol/L, and the content of n-butyllithium is 0.2mol) in the reaction flask with a syringe. ), after the completion of the feeding, keep the reaction for 1h (that is, maintain the temperature in the reaction bottle at -40-35°C for 1h), to obtain a lithium ethoxyformyl solution. Under continuous stirring, insert the syringe into the rubber stopper and slowly add 27.8g (0.21mol) trifluoroacetyl chloride to the reaction flask containing the above obtained ethoxyformyl lithium solution, control the internal temperature of the reaction flask between -10~-5 °C, keep warm for 1h after feeding (that is, maintain the temperature in the reaction flask at -10~-5 °C, 1h), then increase the temperature to 20~25 °C, and continue to stir the reaction for 5h.Post-treatment: the obtained reactants were sequentially subjected to room temperature vacuum filtration, rotary evaporation concentration and vacuum rectification to obtain 32.6g of product
References:
CN115340455,2022,A Location in patent:Paragraph 0067-0092

94726-00-8
35 suppliers
$45.00/10mg

13081-18-0
314 suppliers
$6.00/5g

10186-66-0
37 suppliers
$45.00/50mg

13081-18-0
314 suppliers
$6.00/5g

26728-43-8
0 suppliers
inquiry

13081-18-0
314 suppliers
$6.00/5g