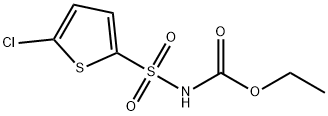
Ethyl5-chlorothiophen-2-ylsulphonylcarbamate synthesis
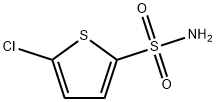
- Product Name:Ethyl5-chlorothiophen-2-ylsulphonylcarbamate
- CAS Number:849793-87-9
- Molecular formula:C7H8ClNO4S2
- Molecular Weight:269.73
Yield:849793-87-9 87%
Reaction Conditions:
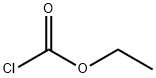
Stage #1: 2-chlorothiophene-5-sulfonamide;chloroformic acid ethyl esterwith caesium carbonate in tetrahydrofuran at 0 - 20; for 36 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;ethyl acetate; pH=~ 1;
Steps:
1.3
A 2-L 3-necked R.B. flask, equipped with a mechanical stirrer and a dropping funnel, was charged with sulfonamide (60.0 g, 303.79 mmol), and Cs2CO3 (20Og, 613.83 mmol, 2.02 equiy) in THF (900 mL). The clear solution was cooled in ice, and ethyl chloroformate (70.0 mL, 734.70 mmol, 2.418 equiv) was added over ca. 30 mins. The heavy suspension was then stirred at room temperature for ca. 36 h.[0107] Then the mixture was diluted with water (200 mL) to yield a clear colorless solution, which was concentrated on rotary evaporator to one-third its volume. This was then diluted with EtOAc (250 mL), cooled in ice, and acidified with 6N HCl to pH ca. 1. The biphasic mixture was transferred to a separatory funnel, layers were separated, and the aqueous layer was again extracted with 2 x 75 mL EtOAc. The combined organic extract was washed with water/brine (2 x 50 mL), brine (1 x 50 mL), dried over Na2SO4, and concentrated to yield the title compound as lightly colored oil. This was purified by filtration through a silica-gel plug. The crude product was applied to the silica-gel plug on a sintered funnel in EtOAc, and then was eluted with EtOAc (1 liter). Concentration of the EtOAc filtrate provided the title compound 8 as a colorless solid, 71.28 g (87%). MS (M-H): 268.0; 270.0. 1H NMR (DMSO): δ 7.62 (d, IH), 7.25 (d, IH), 4.10 (q, 2H), 1.16 (t, 3H).
References:
WO2007/56167,2007,A2 Location in patent:Page/Page column 35