
etonitazene synthesis
- Product Name:etonitazene
- CAS Number:911-65-9
- Molecular formula:C22H28N4O3
- Molecular Weight:396.48
55154-71-7
1 suppliers
inquiry

911-65-9
1 suppliers
$75.00/1mg
Yield: 62.7%
Reaction Conditions:
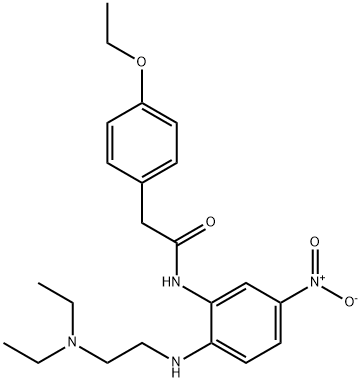
Stage #1:N-[2-{(2-(diethylamino)ethyl)amino}-5-nitrophenyl]-2-(4-ethoxyphenyl)acetamide with phosphorus pentachloride in chloroform for 4 h;Heating / reflux;
Stage #2: with ammonia in chloroform;water; pH=9 - 10 at 20;
Steps:
1
Preparation of 7a-z: N-[2-(2-Diethylamino-ethylamino)-5-nitro-phenyl]-2-(4-ethoxy-phenyl)-acetamide 6a (295 mg, 0.712 mmol), Phosphorous pentachloride (148.3 mg, 0.712 mmol) were dissolved in anhydrous chloroform (10 mL) in a small, argon purged flask fitted with a condenser and magnetic stirbar. The solution was heated to reflux in an oil bath for 4 hours and cooled to room temperature overnight. The mixture was diluted with H2O and chloroform and 2M ammonium hydroxide solution added to adjust pH to 9-10. The mixture was transferred to a separatory funnel and the organic layer collected. The aqueous layer was further extracted with chloroform and the combined organic layers were washed with brine, dried over magnesium sulphate, filtered and concentrated to afford crude. The product was purified using dry silica gel column chromatography eluding with 25 mL portions of solvent system (2.5% 2M NH3 in methanol/95% dichloromethane) to afford a yellow residue. Recrystallization from diethyl ether/hexanes at 0° C. yielded a pale yellow solid 7a {2-[2-(4-Ethoxy-benzyl)-5-nitro-benzoimidazol-1-yl]-ethyl}-diethyl-amine. Yield: 177 mg (62.7%). 1H NMR (DMSO) δ: 0.71 (t, 6H, J=7.0), 1.29 (t, 3H, J=7.0), 2.37 (q, 4H, J=7.1), 2.50 (m, 2H), 3.98 (q, 2H, J=6.9), 4.25 (m, 2H), 4.32 (s, 2H), 6.87 (d, 2H, J=8.4), 7.19 (d, 2H, J=8.4), 7.71 (d, 1H, J=8.9), 8.14 (dd, 2H, J=8.9, 2.1), 8.45 (d, 1H, J=2.1); MS (ESI): 397 (MH+, 100%)
References:
Renton, Paul;Maddaford, Shawn;Rakhit, Suman;Andrews, John US2008/214613, 2008, A1 Location in patent:Page/Page column 40
![1-N-[2-(DIETHYLAMINO)ETHYL]-4-NITROBENZENE-1,2-DIAMINE](/CAS/20180808/GIF/5099-39-8.gif)
5099-39-8
3 suppliers
inquiry

911-65-9
1 suppliers
$75.00/1mg

56223-91-7
9 suppliers
$50.00/50mg

911-65-9
1 suppliers
$75.00/1mg
100-36-7
309 suppliers
$14.14/5gm:

911-65-9
1 suppliers
$75.00/1mg
97-00-7
12 suppliers
$17.67/10gm:

911-65-9
1 suppliers
$75.00/1mg