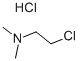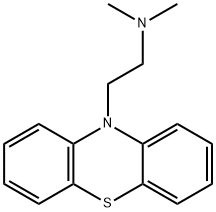
fenethazine synthesis
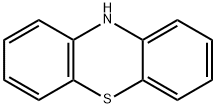
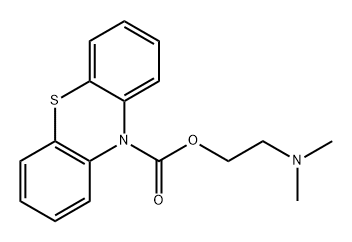
- Product Name:fenethazine
- CAS Number:522-24-7
- Molecular formula:C16H18N2S
- Molecular Weight:270.398
Yield:522-24-7 83%
Reaction Conditions:
Stage #1: 10H-phenothiazinewith n-butyllithium in hexane;toluene at -78; for 1 h;Inert atmosphere;
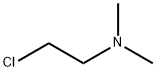
Stage #2: (2-chloroethyl)dimethylamine hydrochloridewith n-butyllithium;triisobutylaluminum in hexane;toluene;Reflux;Inert atmosphere;
Steps:
4.2. Spacer attachment (method A)
General procedure: n-BuLi (1.6M in hexane) was added slowly at-78°C under an inert argon atmosphere in dry toluene (70mL) to a solution of the starting amine and stirred for 1h. Then n-BuLi (1.6M in hexane) and triisobutylalumane (TIBA) were added slowly under argon at-78°C to a stirring solution of 2-chloro-N,N-dimethyl-ethanamine hydrochloride (10) or 3-chloro-N,N-dimethyl-propanamine hydrochloride (56) in dry toluene (30mL), stirring continued 1hat-78°C and added slowly to the previously prepared solution of the starting amine; the resulting mixture was refluxed over night. The crude product was obtained after cooling using ice and the product filtered off to remove tetrabutylammonium chloride (TBACl)/TIBA. The residue was extracted with a mixture of ethyl acetate (100mL) and saturated NaHCO3 solution (20mL), the organic phase washed with 10mL brine, dried with anhydrous Na2SO4 and concentrated under reduced pressure to obtain a colored, oily product. Finally the oily product was purified by column chromatography (silica gel 60 (0.063-0.2 mm/70-230 mesh ASTM), chloroform-methanol 10:1 and 5:1 (v/v)).
References:
Blaess, Markus;Bibak, Nelly;Claus, Ralf A.;Kohl, Matthias;Bonaterra, Gabriel A.;Kinscherf, Ralf;Laufer, Stefan;Deigner, Hans-Peter [European Journal of Medicinal Chemistry,2018,vol. 153,p. 73 - 104] Location in patent:supporting information
18956-87-1
83 suppliers
$6.00/1g

522-24-7
4 suppliers
inquiry