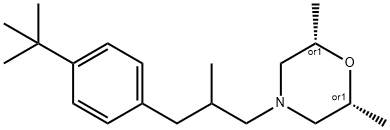
Fenpropimorph synthesis
- Product Name:Fenpropimorph
- CAS Number:67564-91-4
- Molecular formula:C20H33NO
- Molecular Weight:303.48
594-36-5
76 suppliers
$28.00/5g
78613-35-1
112 suppliers
inquiry

67564-91-4
112 suppliers
$65.00/25mg
Yield:67564-91-4 0.14%
Reaction Conditions:
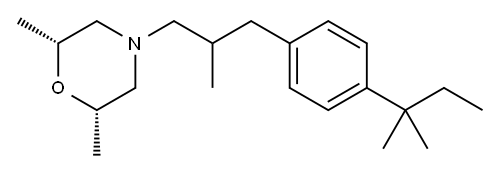
Stage #1:(2R,6S)-2,6-dimethyl-4-(2-methyl-3-phenylpropyl)morpholine hydrochloride with iron(III) chloride in dichloromethane
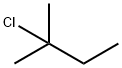
Stage #2:2-methyl-2-butylchloride in dichloromethane at -52 - -20;
Stage #3: with sodium hydroxide;waterProduct distribution / selectivity;more than 3 stages;
Steps:
2a
a) General considerations:1 part bepromoline.HCl is treated with 1.3 parts FeCl3 +5% in dichloromethane at room temperature. The resulting slurry is cooled to approximately - 15 500C, whereupon 1 to 1.1 parts of 2-chloro-2- methylbutane is added.After an appropriate reaction time of around 2.5 hours, the reaction mixture is poured onto an ice-20. water mixture. The organic phase is separated and washed with acidic water, and then with sodium phosphate solution and with sodium hydroxide solution. After a stripping with toluene, extractions with water are performed. The solvent25 is then removed. Then the residue is distilled.Schematic of Production of Amorolfine Base:M MoI. Wt.: 106.59 EPO
References:
GALDERMA S.A. WO2007/12983, 2007, A2 Location in patent:Page/Page column 13-16
141-91-3
123 suppliers
$25.00/5mL
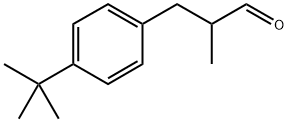
80-54-6
327 suppliers
$9.00/25g

67564-91-4
112 suppliers
$65.00/25mg
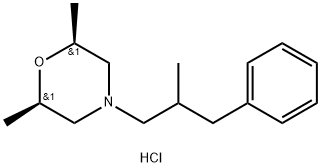
922734-43-8
38 suppliers
inquiry

594-36-5
76 suppliers
$28.00/5g

78613-35-1
112 suppliers
inquiry

67564-91-4
112 suppliers
$65.00/25mg