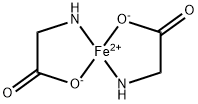
Ferrous Bisglycinate synthesis
- Product Name:Ferrous Bisglycinate
- CAS Number:20150-34-9
- Molecular formula:C4H8FeN2O4
- Molecular Weight:203.96

1345-25-1
126 suppliers
$81.40/5g

6000-44-8
285 suppliers
$16.96/10gm:

20150-34-9
272 suppliers
inquiry
Yield:-
Reaction Conditions:
with citric acid in water at 55 - 60; for 1 h;
Steps:
2
One mole of ferrous oxide is reacted with two moles of sodium glycinate in the presence of citric acid in an aqueous solution to form ferrous bisglycinate. The ferrous bisglycinate is further reacted in a 1:1 molar ratio with citric acid to form ferrous bisglycinate citric acid chelate. Specifically, two moles of sodium glycinate (194.1 g) were dissolved in one liter of 0.5 M citric acid, and the mixture was brought to 55-60° C. Next, 1.0 mole (71.8 g) of ferrous oxide was added to the mixture, and the mixture was allowed to react for 1 hour forming ferrous bisglycinate. Next, one mole of citric acid (192.1 g) was added to the reaction mixture. After a 4 hour reaction time, the composition was cooled 40° C. and spray dried to obtain about 394.0 g of ferrous bisglycinate citrate at 100% yield.
References:
Ashmead, H. DeWayne US2007/270591, 2007, A1 Location in patent:Page/Page column 5