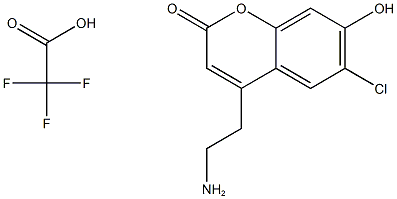
FFN102 synthesis
- Product Name:FFN102
- CAS Number:1234064-11-9
- Molecular formula:C13H11ClF3NO5
- Molecular Weight:353.6783

95-88-5
400 suppliers
$9.00/25g
141734-32-9
12 suppliers
inquiry

76-05-1
676 suppliers
$9.00/1g

1234064-11-9
14 suppliers
$32.00/1mg
Yield:1234064-11-9 37%
Reaction Conditions:
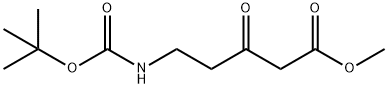
Stage #1: 4-Chlororesorcinol;5-((tert-butoxycarbonyl)amino)-3-oxopentanoic acid methyl esterwith methanesulfonic acid at 0; for 3 h;
Stage #2: trifluoroacetic acid
Steps:
1; S1
To a mixture of 4-chlororesorcinol (0.17 g, 1.2 mmol) and 1 (0.20 g, 0.8 mmol) was added methanesulfonic acid (1.3 mL, 20 mmol) at 0 °C. The clear brown solution gradually became dark orange within 3 h at which point the reaction mixture was diluted with cold ethyl ether (-20 °C, 10 mL), and centrifuged (3000 rpm) at 4 °C for 20 min. After removing the ether solvent by aspiration, the residual orange solid was dried under high vacuum, dissolved in ?0 (3 mL), and purified by RP-HPLC using an appropriate linear gradient of acetonitrile containing 10 % de-ionized water (A) and 0.1 % (v/v) TFA H20. (B) (3-50 % A over 20 min. followed by a steep gradient to 100 % A and equilibrium back to 3 % A). The fractions containing the product (retention time - 12.7 min) were collected, concentrated, and lyophilized to give Mini 102 as a white solid (37%). NMR (DMSO-J6, 300 MHz): δ 11.55 (1H, bs), 7.86 (1H, s), 7.86 (3H, bs), 6.95 (1H, s), 6.25 (1H, s), 3.11 (2H, t, J - 6.2 Hz ), 3.03 (2H, t, ./ = 6.0 Hz). 13C NMR (DMSO-d6, 75 MHz): δ 160.5, 157.4, 154.3, 151.9, 126.4, 118.0, 1 13.4, 1 12.5, 104.5, 38.4, 29.7. LRMS (APCI+): Calc'd for CI IH10C1NO3 239.0 m z, measured 240.2 (MH+).
References:
WO2011/94560,2011,A1 Location in patent:Page/Page column 49; 50