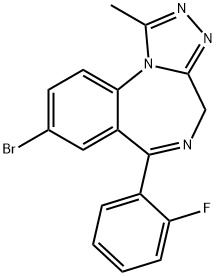
FlubroMazolaM synthesis
- Product Name:FlubroMazolaM
- CAS Number:612526-40-6
- Molecular formula:C17H12BrFN4
- Molecular Weight:371.21
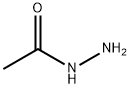
1068-57-1
387 suppliers
$6.00/5g
2647-50-9
2 suppliers
$39.00/1mg

612526-40-6
2 suppliers
$65.00/1mg
Yield:612526-40-6 40%
Reaction Conditions:
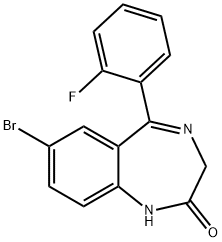
Stage #1:7-bromo-5-(2-fluorophenyl)-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-one with sodium hydride in tetrahydrofuran at 0; for 1 h;
Stage #2: with di-morpholin-4-yl-phosphinic acid chloride in tetrahydrofuran at 0 - 20; for 2 h;
Stage #3:acetic acid hydrazide in tetrahydrofuran;butan-1-ol at 20; for 2.25 h;Heating / reflux;
Steps:
8-Bromo-l-methyl-6- (2'-fluorophenyl)-4H-s-triazolo [4, 3- a] [1, 4] benzodiazepine 16 (JYI-73). A solution of 12 (JYI-032, 7.0 g, 21. 0 mmol) in THF (50 mL) was cooled in ice-water, and sodium hydride (0.72 g, 18 mmol) was added in one portion. After 1 hour, di-4-morpholinylphosphinic chloride (4. 84 g, 22.5 mmol) was added, and the solution which resulted was stirred continuously for 2 hours at room temperature. To this mixture was then added a solution of acetic hydrazide (2.47 g, 30 mmol) in n-BuOH (20 mL) and stirring was continued at room temperature for 15 min. The solvents were evaporated and the residue was dissolved in n-BuOH (25 mL) and heated to reflux for 2 hours. n-Butanol was evaporated and the residue was partitioned between CH2CI2 and brine. The CH2C12 layer was dried and removed under reduced pressure after which the residue was purified by flash chromatography (silica gel, EtOAc) to afford 16 (JYI-73, 2.2 g, 40%) as a white solid: mp 213-214 °C ; IR (KBr) 1610,1484, 1426,1314 cm' ; 1H NMR (DMSO-d6) 6 2.56 (s, 3 H), 4.28 (d, 1 H, J =12. 9 Hz), 5.26 (d, 1 H, J = 12. 9 Hz), 7.24 (t, 1H, J= 8. 3 Hz), 7.29 (t, 1 H, J = 7. 2 Hz), 7.35 (s, 1 H), 7.43-7. 60 (m, 2 H), 7.83 (d, 1 H, J = 8.7 Hz), 7.98 (dd, 1 H, J = 8.7 Hz and 2.3 Hz); MS (EI) m/e (relative intensity) 371 (5), 341 (34), 222 (100), 195 (19), 181 (28), 111 (72). Anal. Calcd. for C17Hl2N4FBr : C, 55.01 ; H, 3.26 ; N, 15.09. Found: C, 54.76 ; H, 3.29 ; N, 14.74.
References:
WISYS TECHNOLOGY FOUNDATION, INC. WO2003/82832, 2003, A2 Location in patent:Page/Page column 46-47

1068-57-1
387 suppliers
$6.00/5g

612526-40-6
2 suppliers
$65.00/1mg