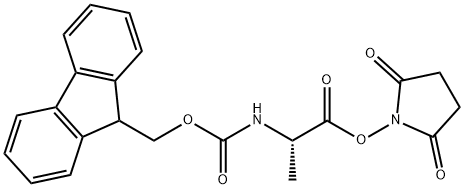
FMOC-ALA-OSU synthesis
- Product Name:FMOC-ALA-OSU
- CAS Number:73724-40-0
- Molecular formula:C22H20N2O6
- Molecular Weight:408.4

35661-39-3
514 suppliers
$5.00/1g

105832-38-0
267 suppliers
$5.00/1g

73724-40-0
88 suppliers
$23.18/1G
Yield: 83%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
5 Step 5. Synthesis Compound 99
To a stirred solution of (2S)-2-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]propanoic acid (Compound 98, 20.00 g, 64.24 mmol, 1.00 equiv) in DMF (200.00 mL) were added TSTU (25.18 g, 83.52 mmol, 1.30 equiv) and DIEA (16.60 g, 128.48 mmol, 2.00 equiv) at room temperature under air atmosphere. The resulting mixture was stirred for 1 h at room temperature. LCMS indicated the reaction was completed. The reaction was diluted with water (200.00 mL), was extracted with EtOAc (100.00 mLx3). The combined organic layer was washed with water (100.00 mL), brine (100.00 mL), dried over anhydrous Na2SO4 and concentrated to dryness in vacuum. The residue was purified by silica gel column chromatography, eluted with (PE: EtOAc =1:2) to give 2,5-dioxopyrrolidin-1-yl (2S)-2-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]propanoate (Compound 99, 25.00 g,83%) t as a white solid. LCMS (ES, m/z):431[M+Na]+.
References:
ORUM THERAPEUTICS, INC. WO2021/198965, 2021, A1 Location in patent:Paragraph 0580; 0610
6066-82-6
744 suppliers
$5.00/10g

35661-39-3
514 suppliers
$5.00/1g

73724-40-0
88 suppliers
$23.18/1G

35661-39-3
514 suppliers
$5.00/1g

73724-40-0
88 suppliers
$23.18/1G